Chemistry
During the chemistry practical when hydrogen sulphide gas having offensive odour is prepared for some test in the laboratory, we can smell the gas from even 50 metres away. Explain the phenomenon.
Answer
The phenomenon is Diffusion.
Diffusion is the process of gradual mixing of two substances, kept in contact, by molecular motion.
The molecules of hydrogen sulphide gas collide with air particles and due to the collisions of the particles, they start moving in a haphazard manner in all possible directions. Due to this, the molecules of hydrogen sulphide gas quickly spread in the surroundings and we can smell the gas from even 50 metres away.
Related Questions
(a) State Charles' law.
(b) Give its
(i) Graphical representation,
(ii) mathematical expression and
(iii) Significance.
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
(a) State the law verified by the following figure:
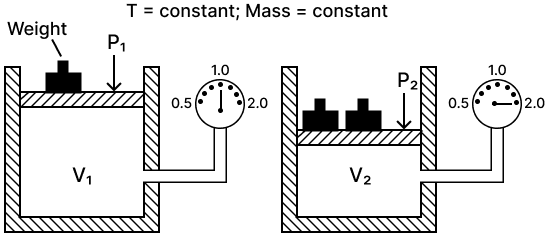
(b) Draw P.V. isothermal for the above law.
(a) State the law which the following graph verifies.
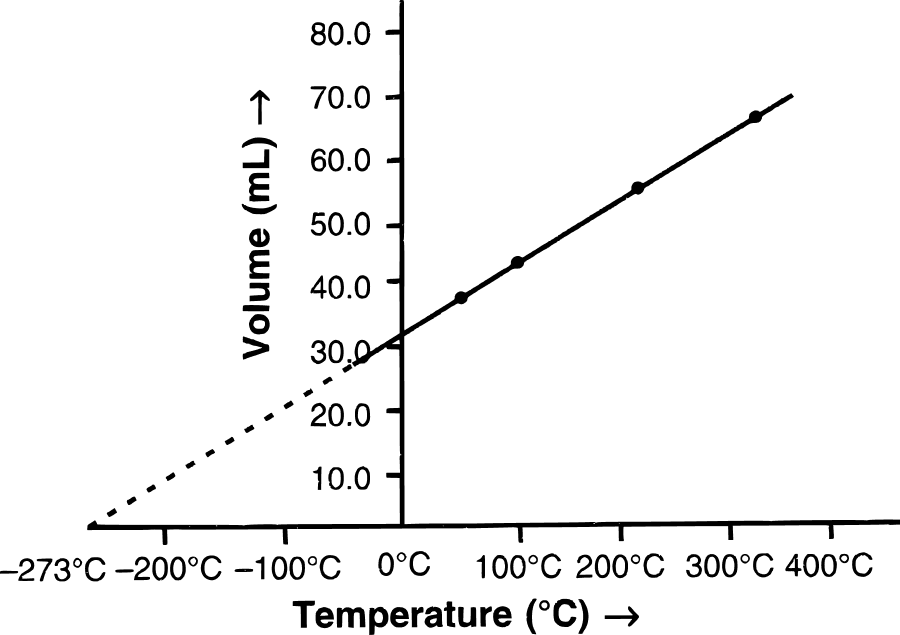
(b) Derive the mathematical expression for it.
(c) Give one application/use where the above law is employed.