Chemistry
(a) State the law which the following graph verifies.
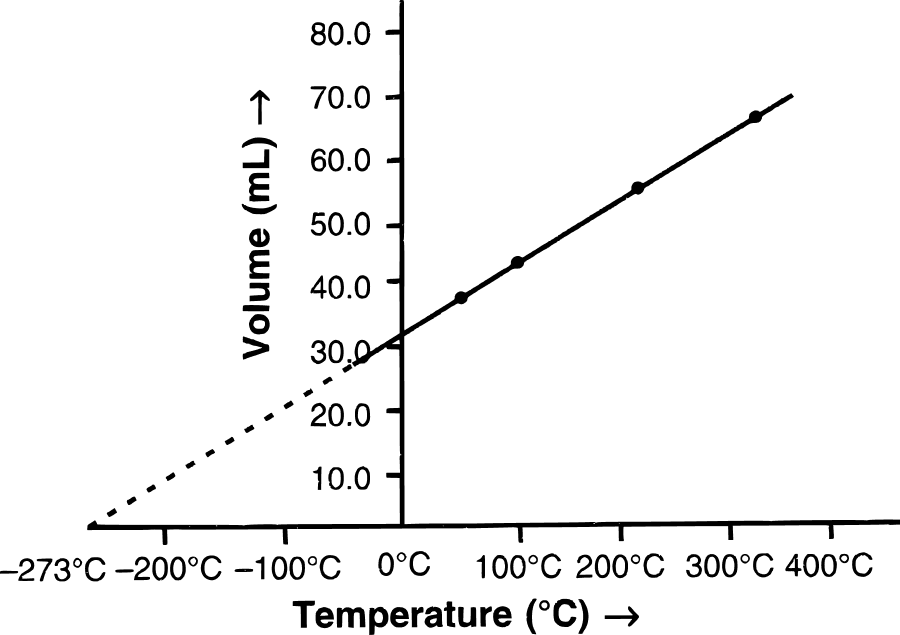
(b) Derive the mathematical expression for it.
(c) Give one application/use where the above law is employed.
Gas Laws
22 Likes
Answer
(a) Charles' law — It states that pressure remaining constant, the volume of a given mass of dry gas increases or decreases by of its volume at 0°C for each 1°C increase or decrease in temperature, respectively.
(b) Let V0 be the volume of a fixed mass of a gas at 0°C and let V be its volume at temperature t°C at constant pressure. Then according to Charles' law,
V = Vo + t (when P is constant)
V = Vo (1 + )
= Vo ()
V = T where T = 273 + t
For a given mass of a gas,
= constant
∴ V = k x T (where k is constant)
or V α T and = K
Suppose a gas occupies V1 cm3 at T1 temperature and V2 cm3 at T2 temperature, then by Charles law,
V1 α T1
or V1 = kT1 (k is constant)
or = k and
V2 α T2
or = k
∴ = = k (at constant pressure)
(c) Volume of a given mass of a gas is directly proportional to its temperature, hence, density decreases with an increase in temperature. This is the reason that hot air is filled into balloons used for meteorological purposes.
Answered By
16 Likes
Related Questions
During the chemistry practical when hydrogen sulphide gas having offensive odour is prepared for some test in the laboratory, we can smell the gas from even 50 metres away. Explain the phenomenon.
(a) State the law verified by the following figure:
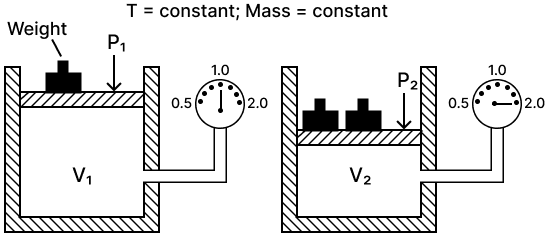
(b) Draw P.V. isothermal for the above law.
Plot V versus absolute T (K) by the given data:
Temperature Volume in litres 27°C 4.8 77°C 5.6 127°C 6.4 177°C 7.2 227°C 8.0 (a) The graph between V and T is a …………… .
(b) Check whether the line passes through the origin.
(c) Which law is obeyed.
Convert :
(i) 273°C to kelvin
(ii) 293 K to °C