Chemistry
(a) State the law verified by the following figure:
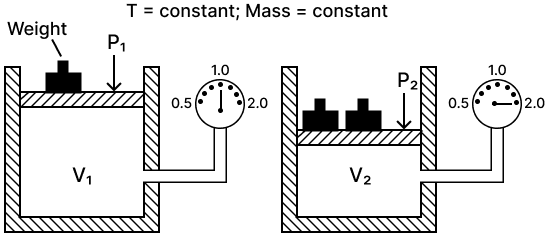
(b) Draw P.V. isothermal for the above law.
Gas Laws
52 Likes
Answer
(a) Boyle's law — It states that the volume of a given mass of a dry gas is inversely proportional to its pressure at a constant temperature i.e., P1V1 = P2V2
(b) P.V. isothermal for Boyle's law is shown below :
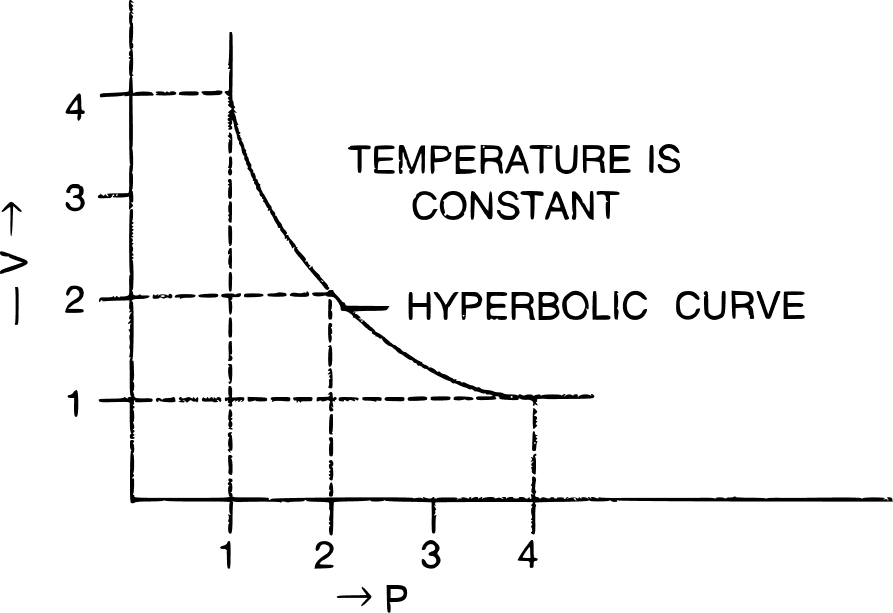
Answered By
39 Likes
Related Questions
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
During the chemistry practical when hydrogen sulphide gas having offensive odour is prepared for some test in the laboratory, we can smell the gas from even 50 metres away. Explain the phenomenon.
(a) State the law which the following graph verifies.
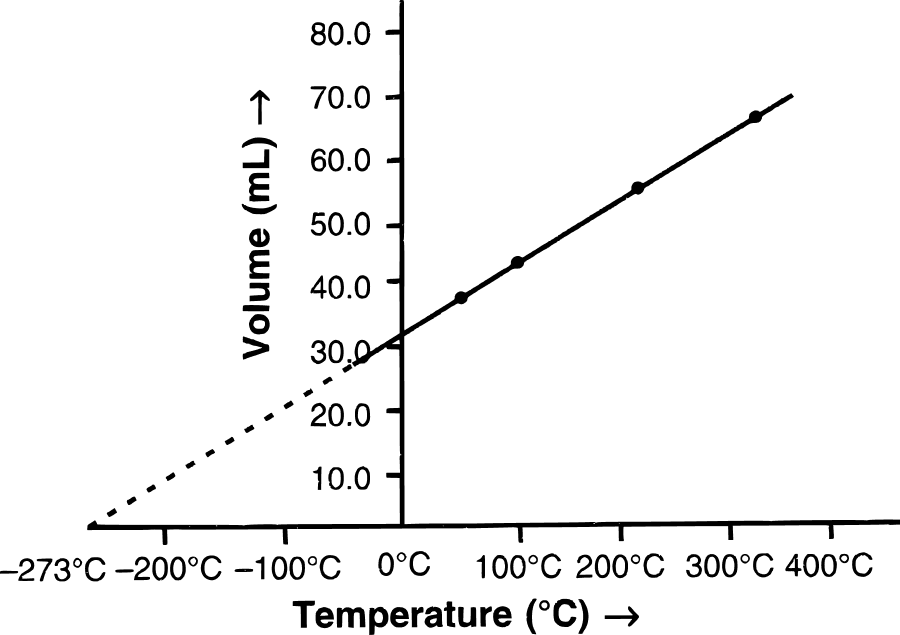
(b) Derive the mathematical expression for it.
(c) Give one application/use where the above law is employed.
Plot V versus absolute T (K) by the given data:
Temperature Volume in litres 27°C 4.8 77°C 5.6 127°C 6.4 177°C 7.2 227°C 8.0 (a) The graph between V and T is a …………… .
(b) Check whether the line passes through the origin.
(c) Which law is obeyed.