Chemistry
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
Answer
When the gas is collected over water, it is moist and contains water vapour. The total pressure exerted by moist gas is equal to the sum of the partial pressure of the dry gas and the pressure exerted by water vapour. The partial pressure of water vapour is called as aqueous tension.
Ptotal = Pgas + Pwater vapour
Pgas = Ptotal – Pwater vapour
Actual pressure of gas = Total pressure – Aqueous tension
Thus, to obtain the actual pressure, the pressure which is due to moisture in the gas has to be subtracted.
Related Questions
(a) State Boyle's Law.
(b) Give its
(i) mathematical expression,
(ii) graphical representation and
(iii) significance.
(a) State Charles' law.
(b) Give its
(i) Graphical representation,
(ii) mathematical expression and
(iii) Significance.
During the chemistry practical when hydrogen sulphide gas having offensive odour is prepared for some test in the laboratory, we can smell the gas from even 50 metres away. Explain the phenomenon.
(a) State the law verified by the following figure:
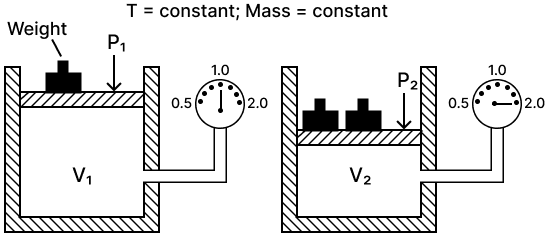
(b) Draw P.V. isothermal for the above law.