Chemistry
Convert :
(i) 273°C to kelvin
(ii) 293 K to °C
Gas Laws
73 Likes
Answer
(i) Kelvin = °C + 273 = 273 + 273 = 546 K
(ii) °C = Kelvin - 273 = 293 - 273 = 20°C
Answered By
45 Likes
Related Questions
(a) State the law which the following graph verifies.
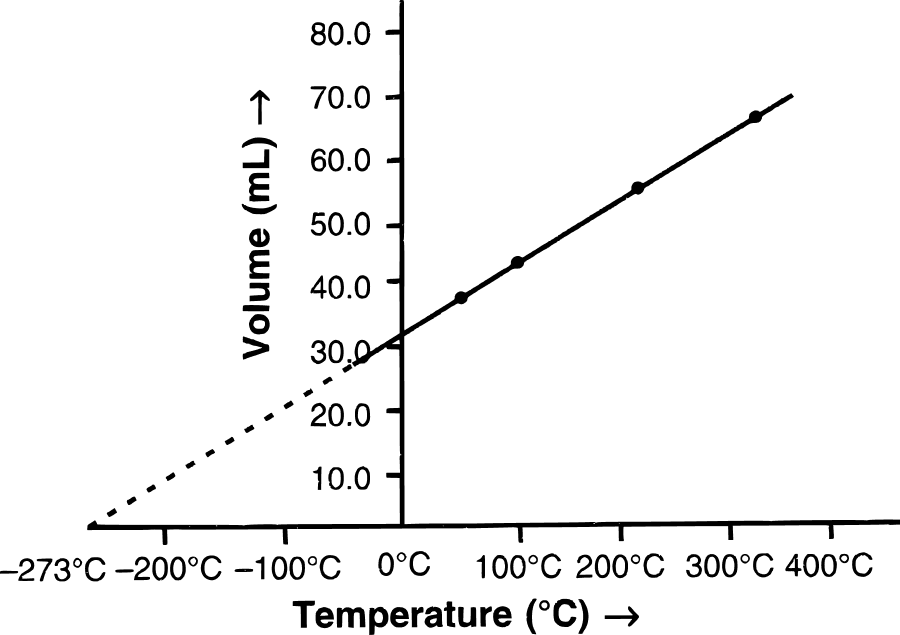
(b) Derive the mathematical expression for it.
(c) Give one application/use where the above law is employed.
Plot V versus absolute T (K) by the given data:
Temperature Volume in litres 27°C 4.8 77°C 5.6 127°C 6.4 177°C 7.2 227°C 8.0 (a) The graph between V and T is a …………… .
(b) Check whether the line passes through the origin.
(c) Which law is obeyed.
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3, if temperature is kept constant?
2 litres of a gas is enclosed in a vessel at a pressure of 760 mm Hg. If temperature remains constant, calculate pressure when volume changes to 4 dm3.