Chemistry
The volume of a given mass of a gas at constant temperature and 10 atmospheric pressure is 10 litres. Its volume at 5 atmospheres will be :
- 5 litres
- 10 litres
- 15 litres
- 20 litres
Gas Laws
40 Likes
Answer
20 litres
Reason — Given,
V1 = 10 l
P1 = 10 atm
P2 = 5 atm
V2 = ?
According to Boyle's law, P1V1 = P2V2
So, 10 x 10 = 5 x V2
V2 = = 20 l
Answered By
29 Likes
Related Questions
The graph shown below gives the statement for :
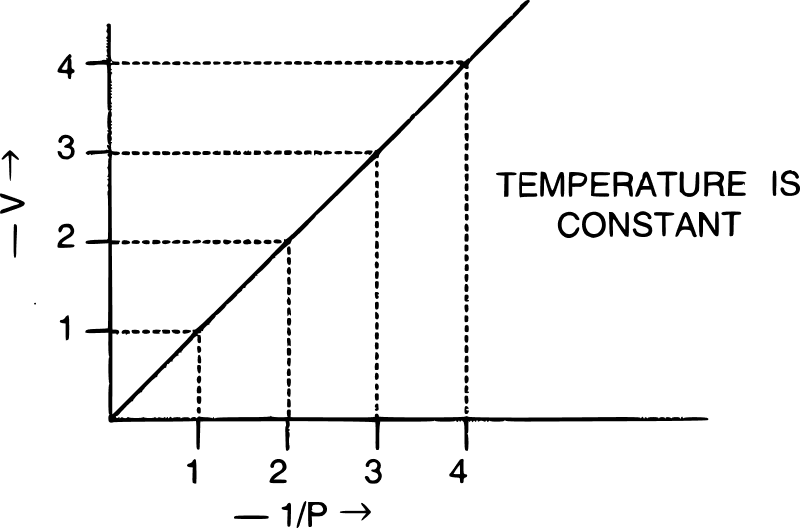
- Henry's law
- Charles' law
- Boyle's law
- Daltons' law
'The volume of a given amount of a gas is directly proportional to its absolute temperature at a constant pressure' is the statement of :
- Gay-Lussac's law
- Boyle's law
- Charles' law
- Mendeleev's law
STP is called standard temperature and pressure. The standard temperature and standard pressure respectively are :
- 273 K and 760 mm
- 0°C and 760 cm
- 273°C and 1 atmosphere
- 373 K and 76 cm
Match the following:
Column A Column A (a) Cm3 (i) Pressure (b) Kelvin (ii) PV = P1V1 (c) Torr (iii) Volume (d) Boyle’s law (iv) = 1}{\text{T}1} (e) Charles law (v) = 1\text{V}1}{\text{T}_1} (vi) temperature