Chemistry
'The volume of a given amount of a gas is directly proportional to its absolute temperature at a constant pressure' is the statement of :
- Gay-Lussac's law
- Boyle's law
- Charles' law
- Mendeleev's law
Gas Laws
24 Likes
Answer
Charles' law
Reason — Charles' law states that volume of a given mass of a dry gas is directly proportional to its absolute (Kelvin) temperature, if the pressure remains constant.
Answered By
15 Likes
Related Questions
Which of the following temperatures is known as the steam point ?
- 273 K
- 373 K
- 290 K
- 273°C
The graph shown below gives the statement for :
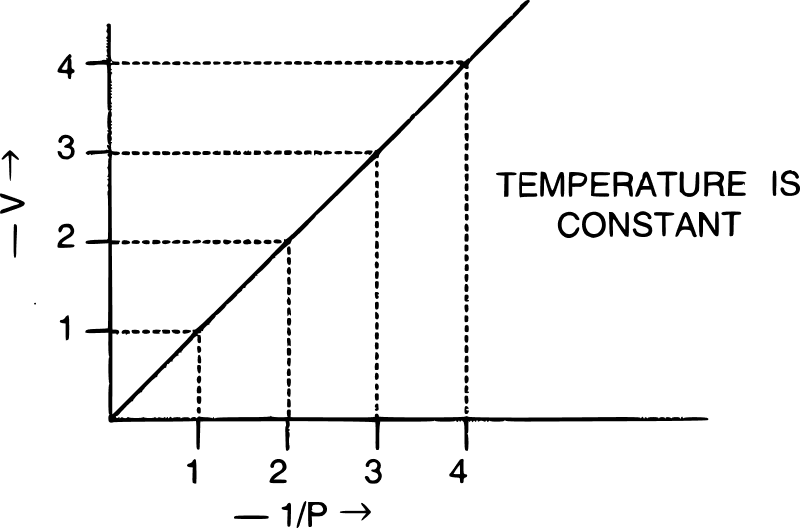
- Henry's law
- Charles' law
- Boyle's law
- Daltons' law
The volume of a given mass of a gas at constant temperature and 10 atmospheric pressure is 10 litres. Its volume at 5 atmospheres will be :
- 5 litres
- 10 litres
- 15 litres
- 20 litres
STP is called standard temperature and pressure. The standard temperature and standard pressure respectively are :
- 273 K and 760 mm
- 0°C and 760 cm
- 273°C and 1 atmosphere
- 373 K and 76 cm