Chemistry
Name or state the following.
- The three isotopes of hydrogen.
- Two elements having same number of protons and electrons but different number of neutrons.
- The valency of an element whose electronic configuration is 2, 8, 3.
- The shell closest to the nucleus of an atom.
- An element having valency zero.
Related Questions
Differentiate between the following terms.
Valence shell and penultimate shell
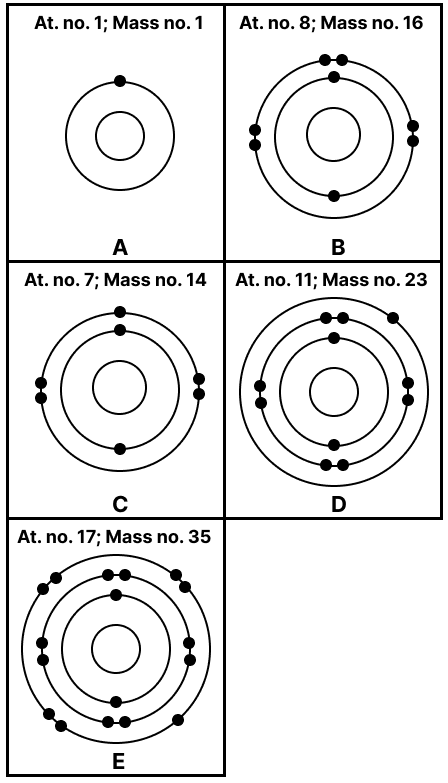
State the number of neutrons in each of the atoms A to E. Also state which of the atoms A to E is a metal.
Differentiate between the following terms.
Octet and duplet
Match the elements A to E in List 1 with their valencies in List 2 and with their nature in List 3.
List 1
[Elements]List 2
[Valency]List 3 A: At. no. 7, Mass no.14 1. -3 X: Metal B: Elec. config. 2,8 2. +1 Y: Non-metal C: Neutrons 14, electrons 13 3. +3 Z: Noble gas D: Neutrons 22, protons 18 4. +2 E: Elec. config. 2,8,1 5. 0