Chemistry
Name or state the following.
- The three isotopes of hydrogen.
- Two elements having same number of protons and electrons but different number of neutrons.
- The valency of an element whose electronic configuration is 2, 8, 3.
- The shell closest to the nucleus of an atom.
- An element having valency zero.
Atomic Structure
31 Likes
Answer
- Protium [], Deuterium [], Tritium []
- Sodium [e = p = 11, n = 12] and Chlorine [e = p = 17, n = 18]
- The valency is 3 positive. Electronic configuration = 2, 8, 3. Therefore, the element tends to lose 3 electrons and gain stable octet configuration.
- K shell.
- Noble gases [He, Ne, Ar etc.]
Answered By
19 Likes
Related Questions
Differentiate between the following terms.
Valence shell and penultimate shell
Differentiate between the following terms.
Octet and duplet
State the number of neutrons in each of the atoms A to E. Also state which of the atoms A to E is a metal.
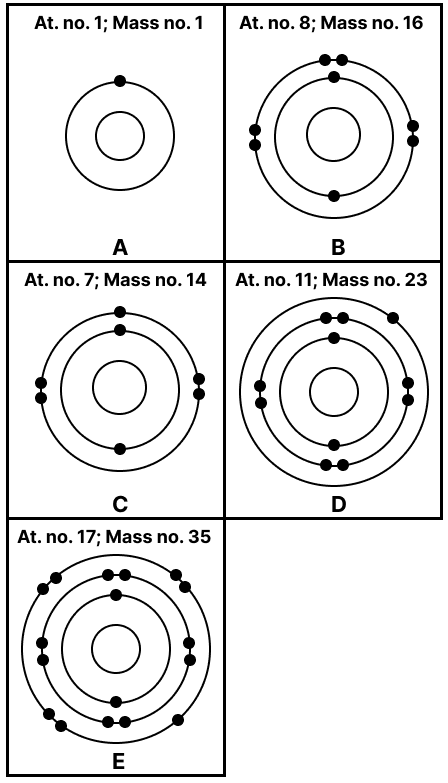
Match the elements A to E in List 1 with their valencies in List 2 and with their nature in List 3.
List 1
[Elements]List 2
[Valency]List 3 A: At. no. 7, Mass no.14 1. -3 X: Metal B: Elec. config. 2,8 2. +1 Y: Non-metal C: Neutrons 14, electrons 13 3. +3 Z: Noble gas D: Neutrons 22, protons 18 4. +2 E: Elec. config. 2,8,1 5. 0