Physics
(i) Represent the change in the nucleus of a radioactive element when a β particle is emitted.
(ii) What is the name given to elements with same mass number and different atomic number?
(iii) Under which conditions does the nucleus of an atom tend to be radioactive?
Radioactivity
ICSE 2016
6 Likes
Answer
(i) If a radioactive nucleus P with mass number A and atomic number Z emits a β particle to form a daughter nucleus Q with mass number A and atomic number Z + 1, the change can be represented as follows:
ii) Elements with same mass number A, but different atomic number Z are called isobars.
iii) The nucleus of an atom tend to be radioactive when the number of neutrons is more than the number of protons. The nucleus thus becomes unstable and in order to gain stability it tends to emit α, β, γ radiations.
Answered By
4 Likes
Related Questions
(i) Name the transformer used in the power transmitting station of a power plant.
(ii) What type of current is transmitted from the power station?
(iii) At what voltage is this current available to our household?
A battery of e.m.f. 12 V and internal resistance 2 Ω is connected with two resistors A and B of resistance 4 Ω and 6 Ω respectively joined in series.
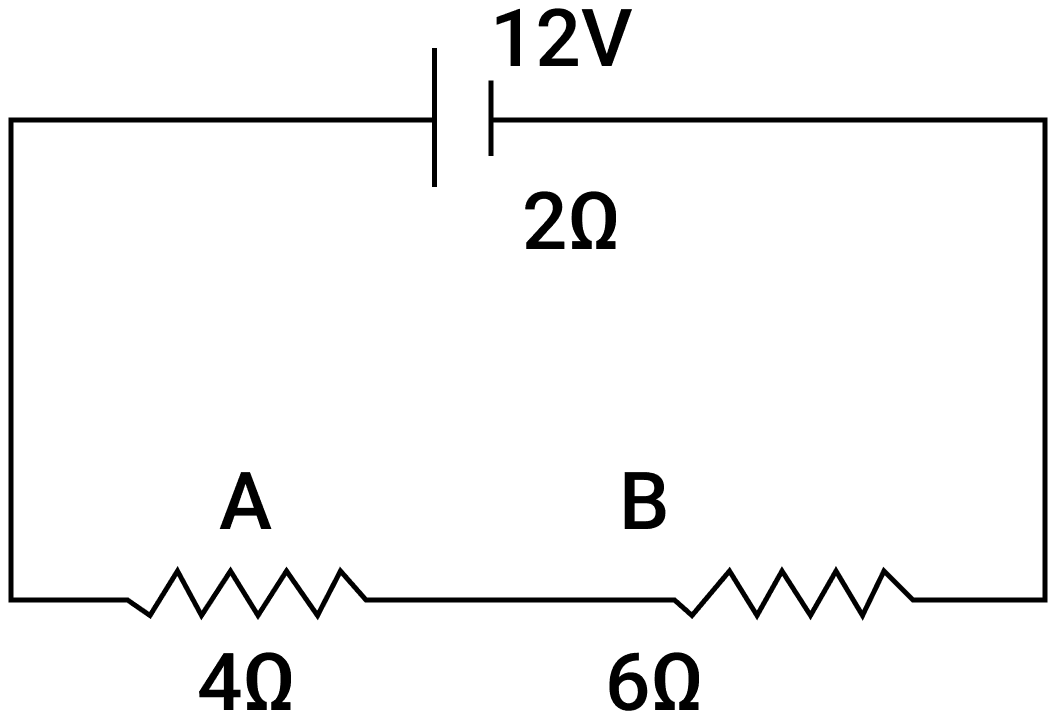
Find:
(i) Current in the circuit.
(ii) The terminal voltage of the cell.
(iii) The potential difference across 6 Ω Resistor.
(iv) Electrical energy spent per minute in 4 Ω Resistor.
Arrange α, β and γ rays in ascending order with respect to their
(i) Penetrating power.
(ii) Ionising power.
(iii) Biological effect.
(i) In a cathode ray tube what is the function of anode?
(ii) State the energy conversion taking place in a cathode ray tube.
(iii) Write one use of cathode ray tube.