Chemistry
(i) Give the IUPAC name of the following organic compounds:
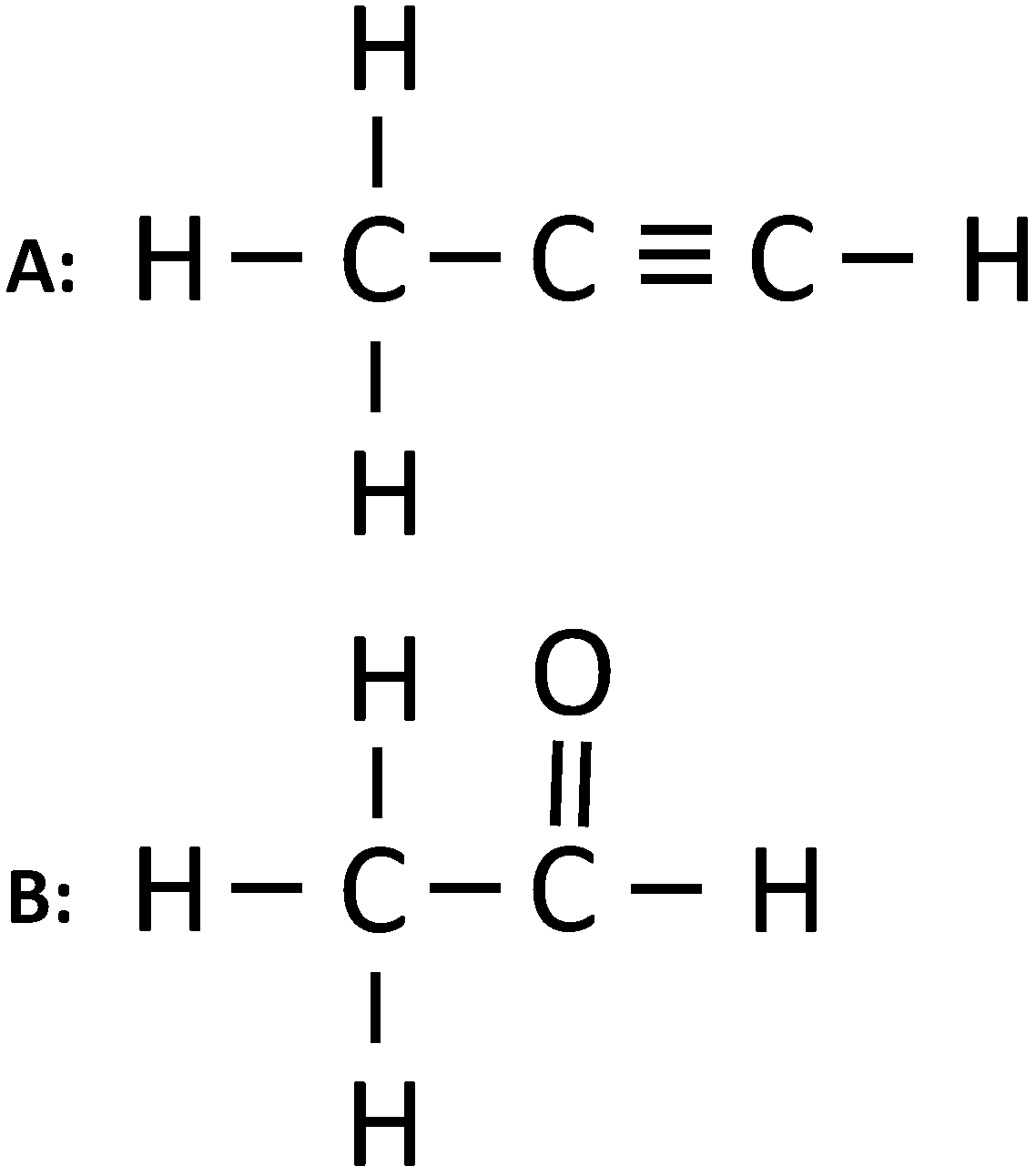
(ii) What is the special feature of the structure of ethyne
(iii) Name the saturated hydrocarbon containing two carbon atoms.
(iv) Give the structural formula of acetic acid.
Answer
(i) The IUPAC names are:
A: Propyne
B: Ethanal
(ii) The special feature of the structure of ethyne is that the two carbon atoms are linked by triple covalent bond formed by sharing three pairs of electrons between the two carbon atoms. The availability of electrons on the triple bond makes ethyne more reactive and hence it undergoes characteristics addition reactions only.
(iii) Ethane [C2H6]
(iv) Structural formula of acetic acid is shown below:

Related Questions
Arrange - Ethane, methane, ethene, ethyne - in increasing order of molecular weight [H = 1, C = 12]
Give balanced chemical equations for the preparation of :
(i) Ethene from bromoethane
(ii) Ethyne using calcium carbide
(iii) Methane from sodium acetate.
Fill in the blanks with the choices given in the brackets:
(i) Conversion of ethanol to ethene by the action of conc. sulphuric acid is an example of …………… [dehydration/dehydrogenation/dehydrohalogenation]
(ii) Substitution reactions are characteristic reactions of ……………[alkynes/alkenes/alkanes].
Write a balanced chemical equation for the following reactions: Chlorine gas is reacted with ethene.