Chemistry
Arrange - Ethane, methane, ethene, ethyne - in increasing order of molecular weight [H = 1, C = 12]
Organic Chemistry
10 Likes
Answer
Ethane [C2H6] : M.W. = 2[12] + 6[1] = 30
Methane [CH4] : M.W. = 12 + 4[1] = 16
Ethene [C2H4] : M.W. = 2[12] + 4[1] = 28
Ethyne [C2H2] : M.W. = 2[12] + 2[1] = 26
Hence, increasing order of molecular weight :
Methane < Ethyne < Ethene < Ethane
Answered By
6 Likes
Related Questions
Write a balanced chemical equation for the following reactions: Chlorine gas is reacted with ethene.
(i) Give the IUPAC name of the following organic compounds:
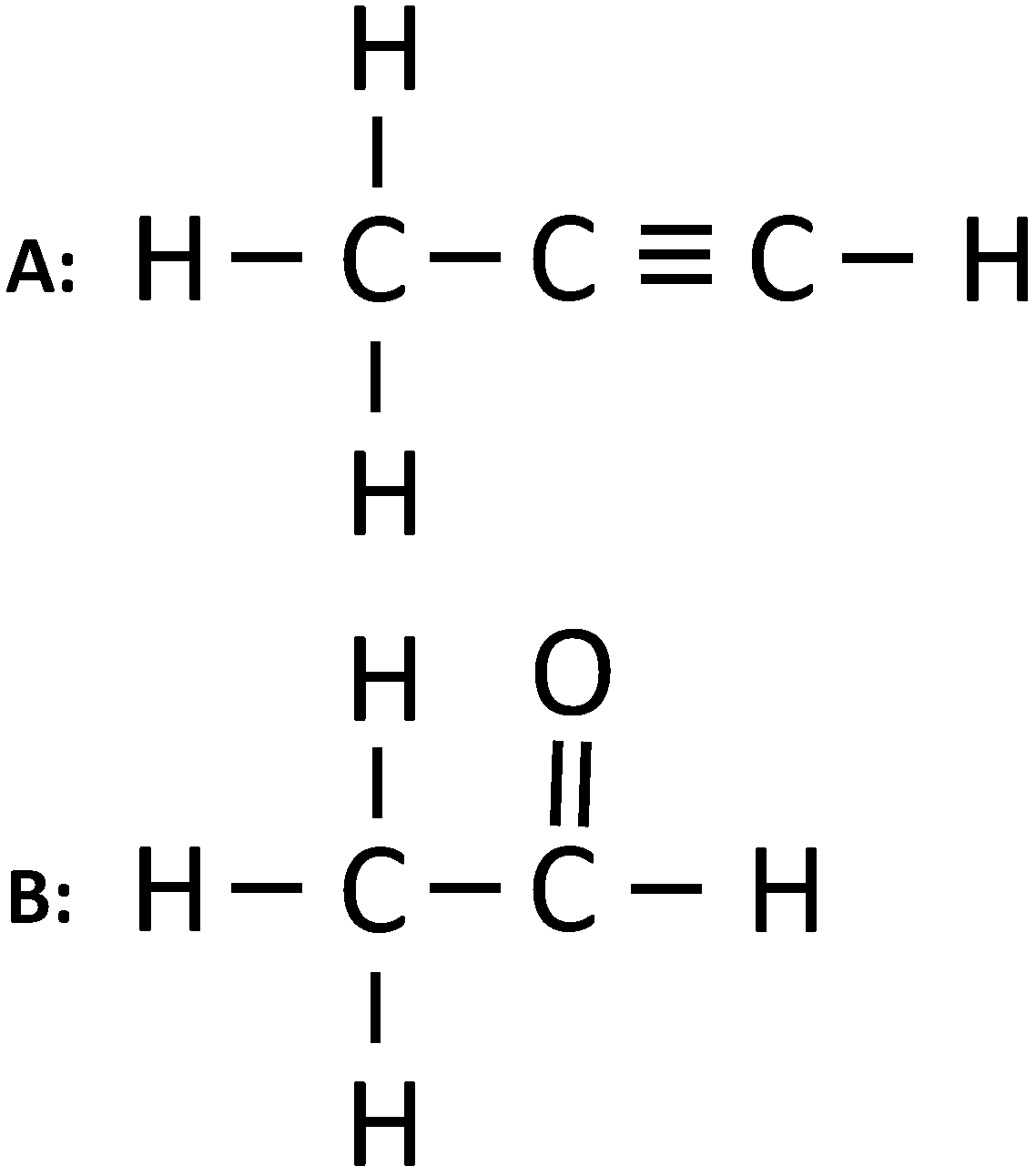
(ii) What is the special feature of the structure of ethyne
(iii) Name the saturated hydrocarbon containing two carbon atoms.
(iv) Give the structural formula of acetic acid.
Give balanced chemical equations for the preparation of :
(i) Ethene from bromoethane
(ii) Ethyne using calcium carbide
(iii) Methane from sodium acetate.
Name the following organic compounds:
(i) The compound with three carbon atoms whose functional group is a carboxyl.
(ii) The first homologue whose general formula is CnH2n
(iii) The compound that reacts with acetic acid to form ethyl ethanoate
(iv) The compound formed by complete chlorination of ethyne.