Chemistry
(a) State Boyle's Law.
(b) Give its
(i) mathematical expression,
(ii) graphical representation and
(iii) significance.
Gas Laws
47 Likes
Answer
(a) Boyle's law states that the volume of a given mass of a dry gas is inversely proportional to its pressure at a constant temperature.
(b) (i) Mathematical expression of Boyle's law :
Suppose a gas occupies volume V1 when its pressure is P1; then
V1 α or
V1 = or
P1V1 = k = constant
If V2 is the volume occupied when the pressure is P2 at the same temperature, then
V2 α or
V2 = or
P2V2 = k = constant
∴ P1V1 = P2V2 = k; at constant temperature.
(ii) Graphical representation of Boyle's Law:
(a) V vs : a straight line passing through the origin is obtained.
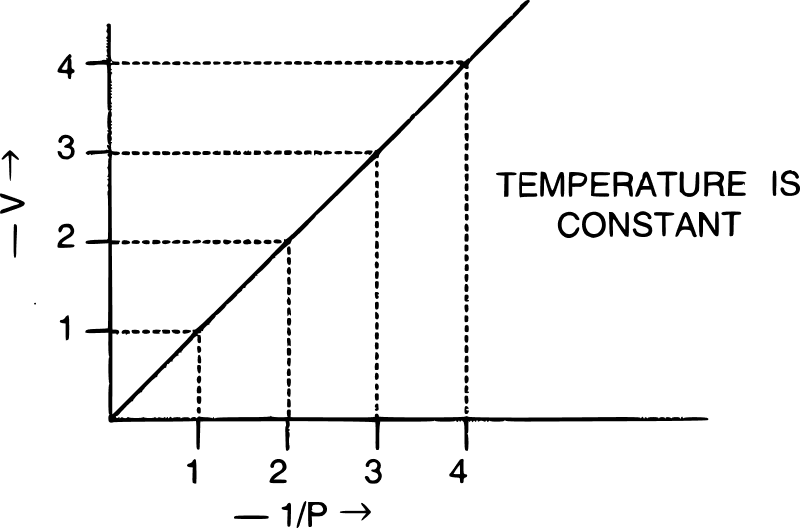
(b) V vs P : a hyperbolic curve in the first quadrant is obtained.
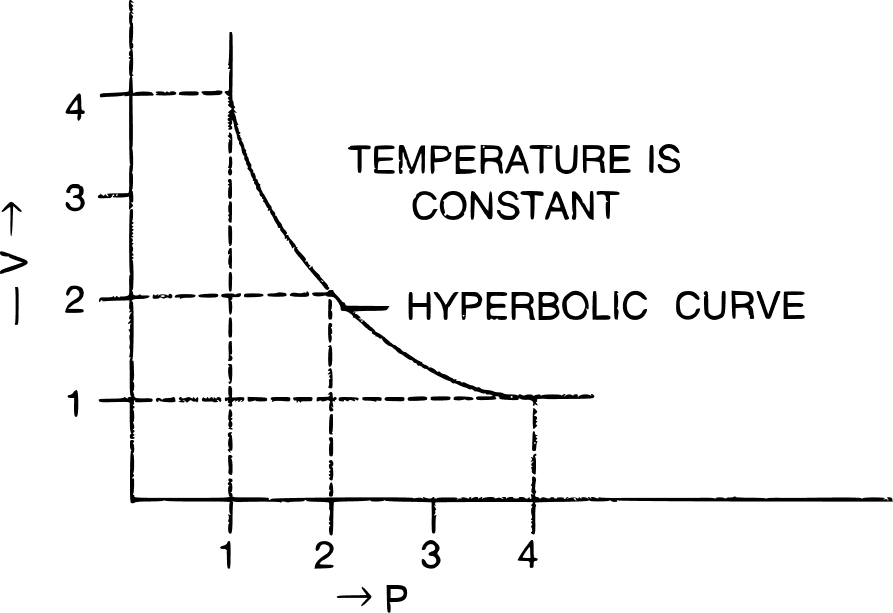
(c) PV vs P : a straight line is obtained parallel to the pressure axis.
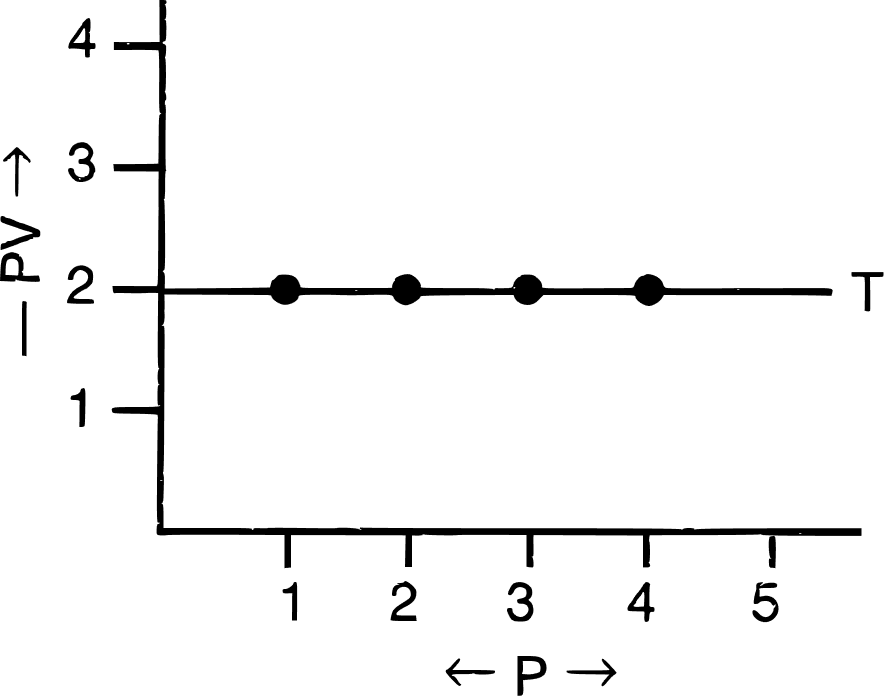
(iii) Significance of Boyle's law :
On increasing pressure, volume decreases. The gas becomes denser. Thus, at constant temperature, the density of a gas is directly proportional to pressure.
Answered By
22 Likes
Related Questions
Explain Charles' law on the basis of the kinetic theory of matter.
How did Charles' law lead to the concept of an absolute scale of temperature?
(a) State Charles' law.
(b) Give its
(i) Graphical representation,
(ii) mathematical expression and
(iii) Significance.
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?