Physics
A radioactive nucleus X emits an alpha particle followed by two beta particles to form nucleus Y.
(a) With respect to the element X, where would you position the element Y in the periodic table?
(b) What is the general name of the element X and Y.
(c) If the atomic number of Y is 80 then what is the atomic number of X?
Radioactivity
ICSE Sp 2024
103 Likes
Answer
(a) AZX ⟶ A-4Z-2X1 + 42He ⟶ A-4Z-1X2 + 0-1e ⟶ A-4ZY + 0-1e
We can see from the above that X and Y have same atomic number. Hence, X and Y will occupy the same position in the periodic table.
(b) Isotope.
(c) When the atomic no of Y = 80 and X and Y are isotopes then atomic no. of X will also be 80.
Answered By
70 Likes
Related Questions
The figure alongside shows a simple pendulum of mass 200 g. It is displaced from the mean position A to the extreme position B. The potential energy at the position A is zero. At the position B the pendulum bob is raised by 5 m.
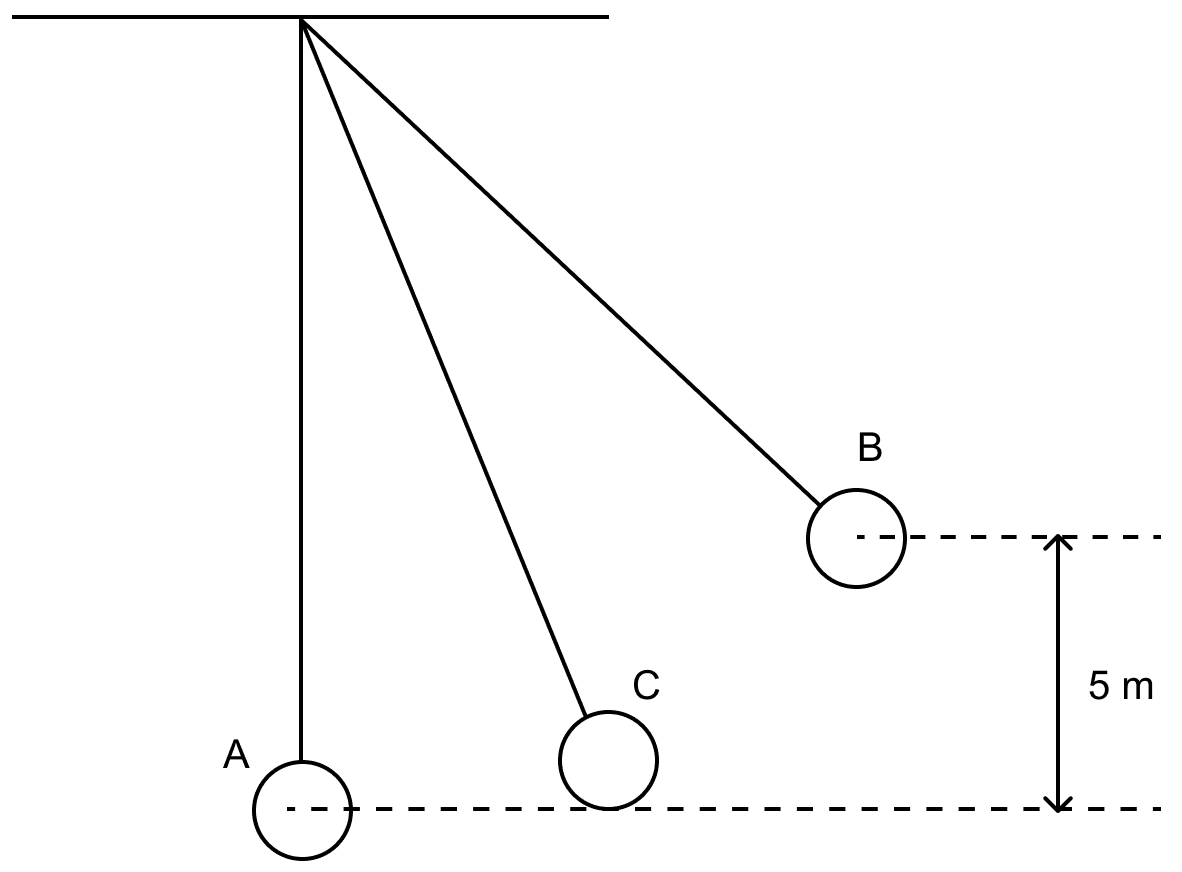
(i) What is the potential energy of the pendulum at the position B?
(ii) What is the total mechanical energy at point C?
(iii) What is the speed of the bob at the position A when released from B?
(Take g = 10 ms-2 and there is no loss of energy.)
A person standing in front of a cliff fires a gun and hears its echo after 3s. If the speed of sound in air is 336 ms-1
(a) Calculate the distance of the person from the cliff.
(b) After moving a certain distance from the cliff, he fires the gun again and this time the echo is heard 1.5 s later than the first. Calculate distance moved by the person.
A boy tunes a radio channel to a radio station 93.5 MHz.
(a) Name and define the scientific wave phenomenon involved in tuning the radio channel.
(b) Name the important characteristics of sound that is affected during this phenomenon.
(c) Convert 93.5 MHz to SI unit.
Purvi's friend Tim wants to connect a fuse to his oven. He wants to control the oven from two different locations. Shown below is his circuit diagram.

(a) Which one of the two, A or B should be a live wire?
(b) In the event of an overload, will the fuse serve its purpose?
(c) What is the meaning of the statement that the bulb is rated 600W, 220 V?