Physics
A nucleus 84X202 of an element emits an alpha particle followed by a beta particle. The final nucleus is aYb. Find a and b.
Radioactivity
ICSE 2020
14 Likes
Answer
The emission of alpha and beta particle is represented below:
∴ a = 83 and b = 198
Answered By
9 Likes
Related Questions
Two metallic blocks P and Q having masses in ratio 2 : 1 are supplied with the same amount of heat. If their temperatures rise by same degree, compare their specific heat capacities.
When a current carrying conductor is placed in a magnetic field, it experiences a mechanical force. What should be the angle between the magnetic field and the length of the conductor so that the force experienced is:
(i) Zero
(ii) Maximum?
The diagram alongside shows a loop of wire carrying current I:

(i) What is the magnetic polarity of the loop that faces us?
(ii) With respect to the diagram how can we increase the strength of the magnetic field produced by this loop?
The figure alongside shows a simple pendulum of mass 200 g. It is displaced from the mean position A to the extreme position B. The potential energy at the position A is zero. At the position B the pendulum bob is raised by 5 m.
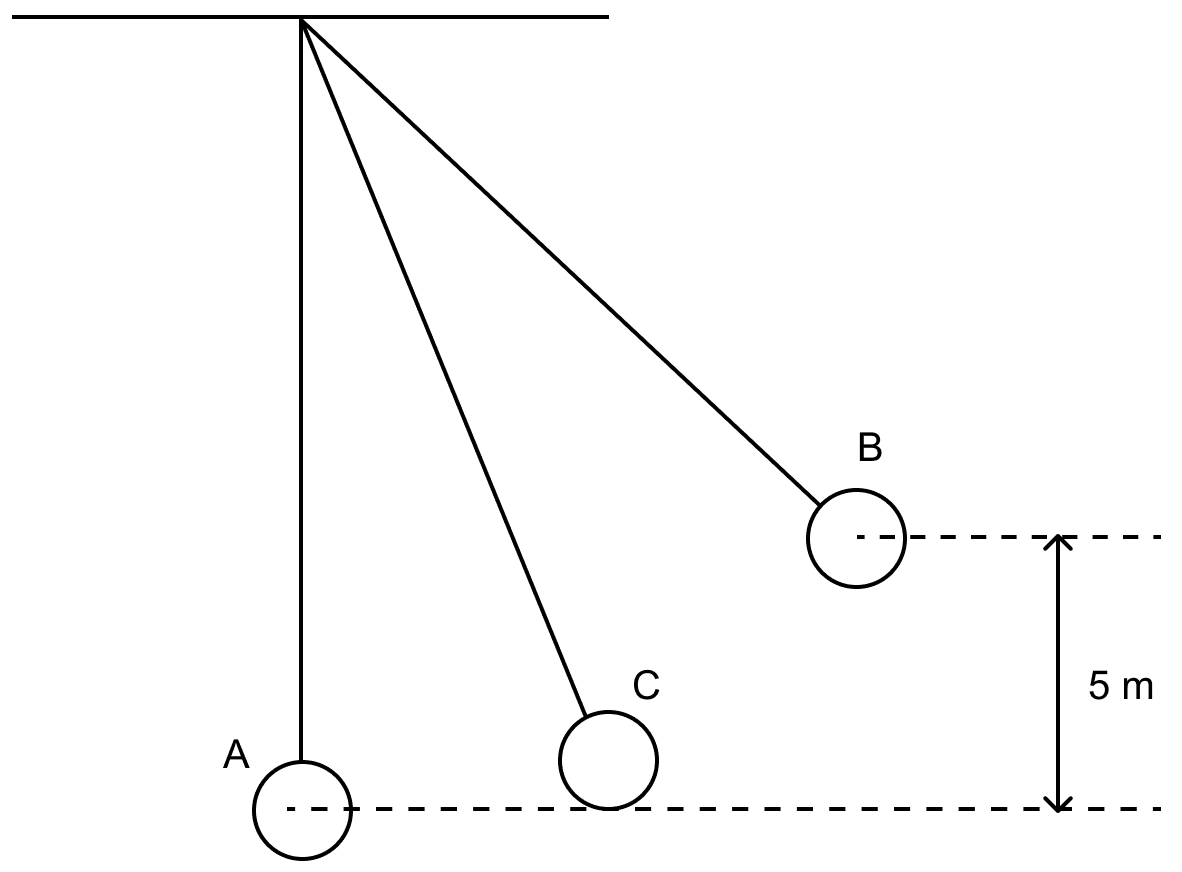
(i) What is the potential energy of the pendulum at the position B?
(ii) What is the total mechanical energy at point C?
(iii) What is the speed of the bob at the position A when released from B?
(Take g = 10 ms-2 and there is no loss of energy.)