Chemistry
Answer
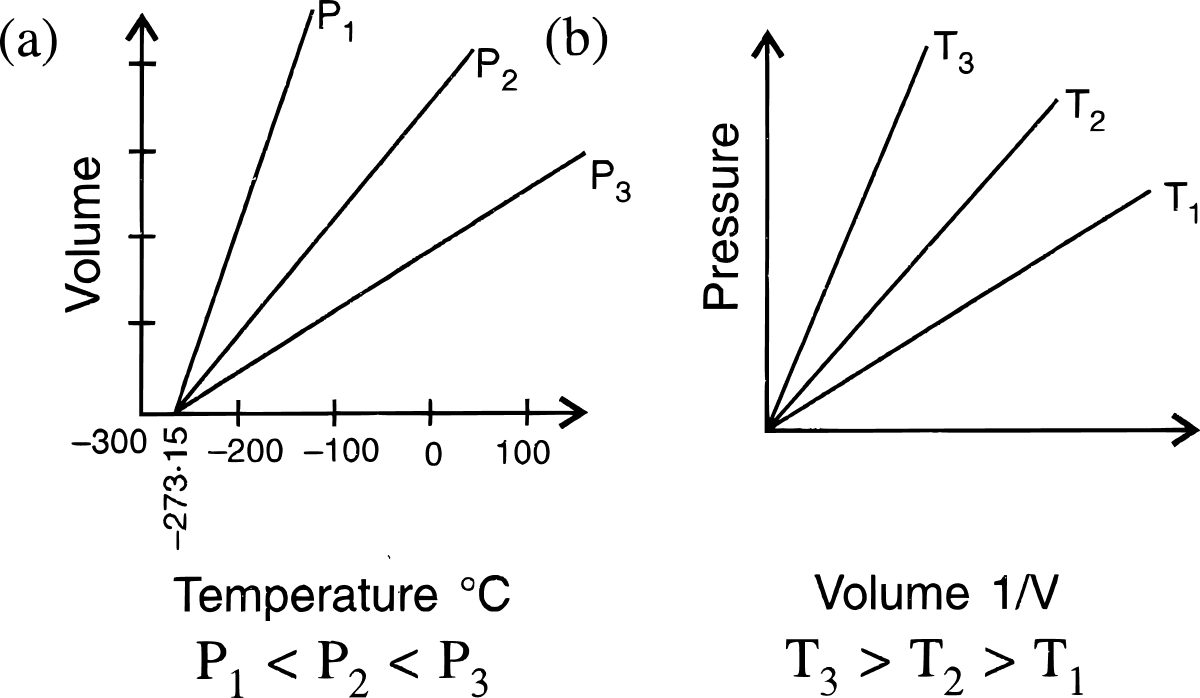
(a) The standard values chosen are 0°C or 273 K for temperature, and 1 atmospheric unit (atm) or 760 mm Hg for pressure. These standard values are known as standard temperature and pressure (S.T.P.).
(b) Since volume of a gas changes remarkably with change in temperature and pressure, it becomes necessary to choose standard values of temperature and pressure to which gas volumes can be referred.