Chemistry
(a) Define S.T.P.
(b) Why is it necessary to compare gases at S.T.P.?
Gas Laws
81 Likes
Answer
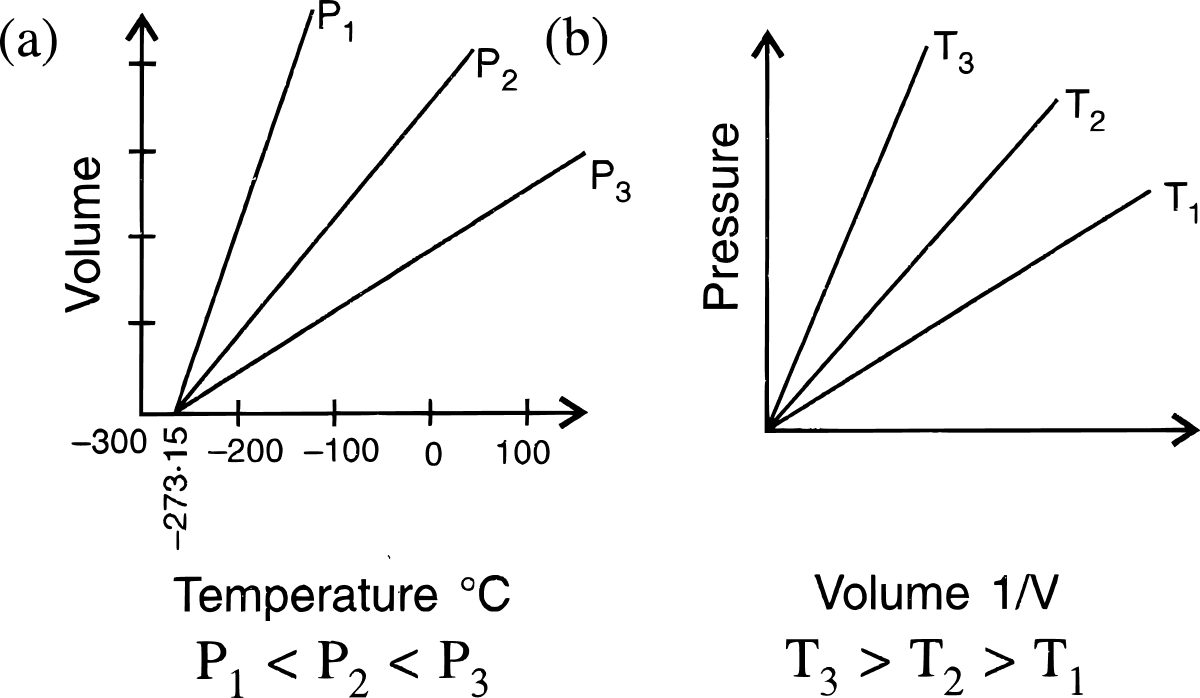
(a) The standard values chosen are 0°C or 273 K for temperature, and 1 atmospheric unit (atm) or 760 mm Hg for pressure. These standard values are known as standard temperature and pressure (S.T.P.).
(b) Since volume of a gas changes remarkably with change in temperature and pressure, it becomes necessary to choose standard values of temperature and pressure to which gas volumes can be referred.
Answered By
55 Likes