Chemistry
The molecular theory states that the pressure exerted by a gas in closed vessel results from the gas molecules striking against the walls of the vessel. How will the pressure change if :
(a) the temperature is doubled keeping the volume constant
(b) the volume is made half of its original value keeping the temperature constant?
Related Questions
State the laws which are represented by the following graphs.
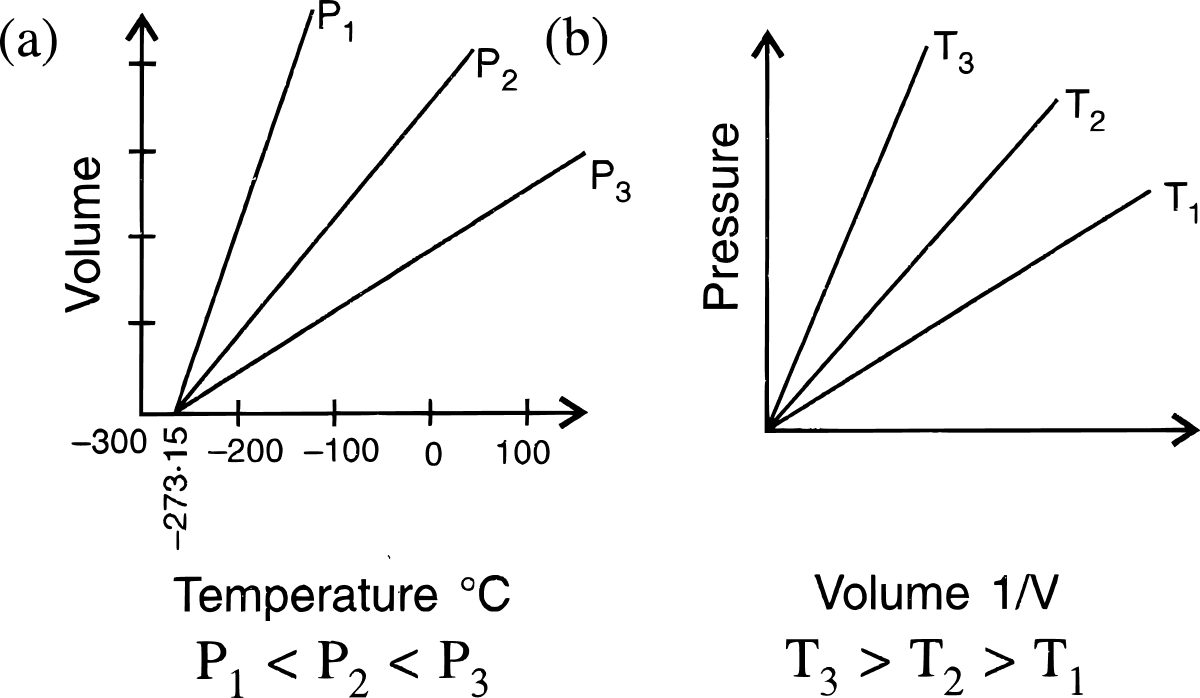
Give reasons for the following :
All temperatures in the absolute (Kelvin) scale are in positive figures.
(a) What is the need for the Kelvin scale of temperature?
(b) What is the boiling point of water on the Kelvin scale? Convert it into centigrade scale.
What is the relationship between the Celsius and the Kelvin scales of temperature?