Chemistry
Oxygen atom in water has two 'lone pair of electrons'. Explain the meaning of the term in italics. With the help of an electron dot diagram show the formation of hydronium ion and ammonium ion from a water molecule and an ammonia molecule respectively.
Acids Bases Salts
53 Likes
Answer
Oxygen atom in water has two 'lone pair of electrons' implies that two pairs of electrons on oxygen are not shared with any other atom as shown below:
Formation of hydronium ion
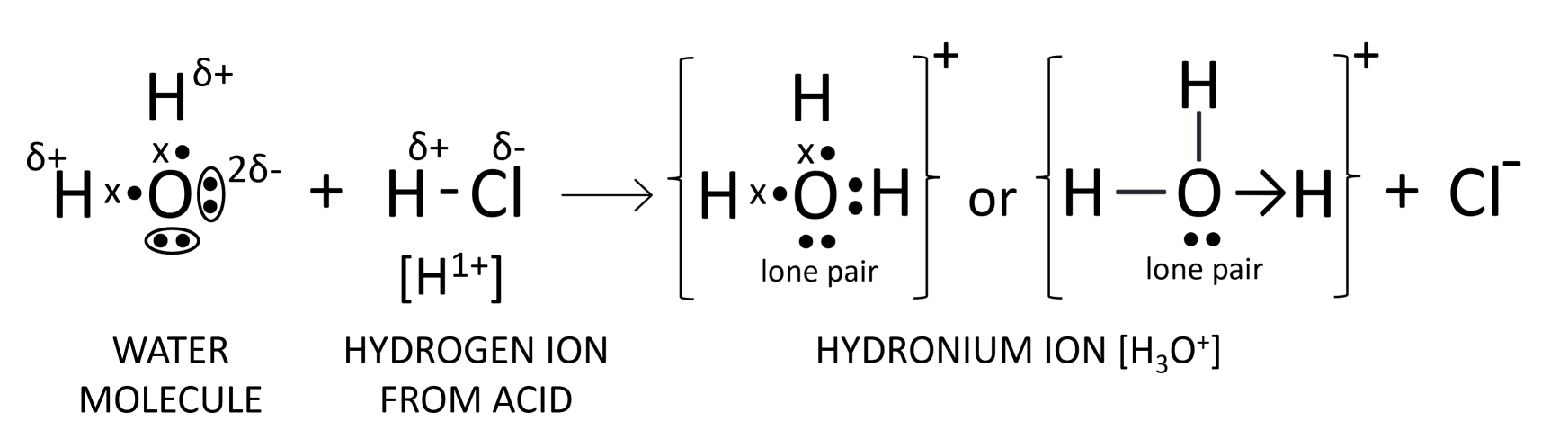
Formation of ammonium ion

Answered By
34 Likes
Related Questions
Give three reasons with equations wherever required, why Sulphuric acid is a dibasic acid.
State how acids are defined as per Arrhenius's and Lowry – Bronsted's theory.
State how you would obtain:
- Sulphuric acid from an acidic oxide
- KOH from a basic oxide.
State two chemical properties each with equations of a solution containing
(i) H+ ions
(ii) OH- ions