Chemistry
(i) What do you understand by a lone pair of electrons?
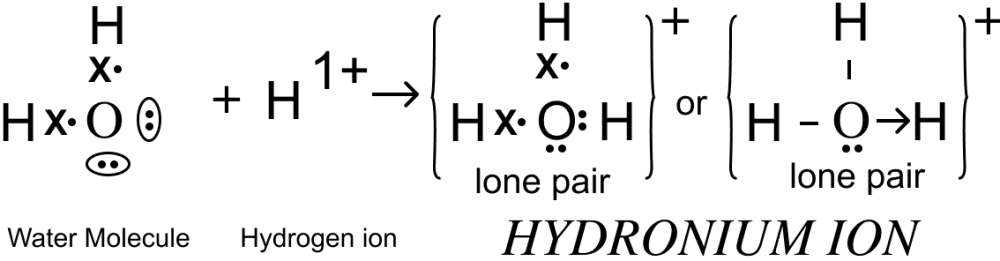
(ii) Draw the electron dot diagram of Hydronium ion. (H=1; O=8)
Chemical Bonding
ICSE 2018
8 Likes
Answer
(i) Lone pair of electrons are valence electrons that in a covalent bond are not shared with another atom.
(ii) Electron dot diagram of Hydronium ion is shown below:
Answered By
6 Likes
Related Questions
Name the gas that is produced in each of the following cases:
(i) Sulphur is oxidized by concentrated nitric acid.
(ii) Action of dilute hydrochloric acid on sodium sulphide.
(iii) Action of cold and dilute nitric acid on copper.
(iv) At the anode during the electrolysis of acidified water.
(v) Reaction of ethanol and sodium.
Fill up the blanks with the correct choice given in brackets.
(i) Ionic or electrovalent compounds do not conduct electricity in their …………… state. (fused / solid)
(ii) Electrolysis of aqueous sodium chloride solution will form …………… at the cathode. (hydrogen gas / sodium metal)
(iii) Dry hydrogen chloride gas can be collected by…………… displacement of air. (downward / upward)
(iv) The most common ore of iron is …………… (calamine / haematite)
(v) The salt prepared by the method of direct combination is …………… (iron (II) chloride / iron (III) chloride)
In Period 3 of the Periodic Table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the brackets to complete the following statements:
(i) The element B would have (lower/higher) metallic character than A.
(ii) The element A would probably have (lesser/higher) electron affinity than B.
(iii) The element A would have (greater/smaller) atomic size than B.
Copy and complete the following table which refers to the conversion of ions to neutral particles.
Conversion Ionic equation Oxidation/Reduction Chloride ion to chlorine molecule Lead [II] ion to lead