Physics
Explain how is the height of mercury column in the tube of a simple barometer, a measure of the atmospheric pressure.
Fluids Pressure
31 Likes
Answer
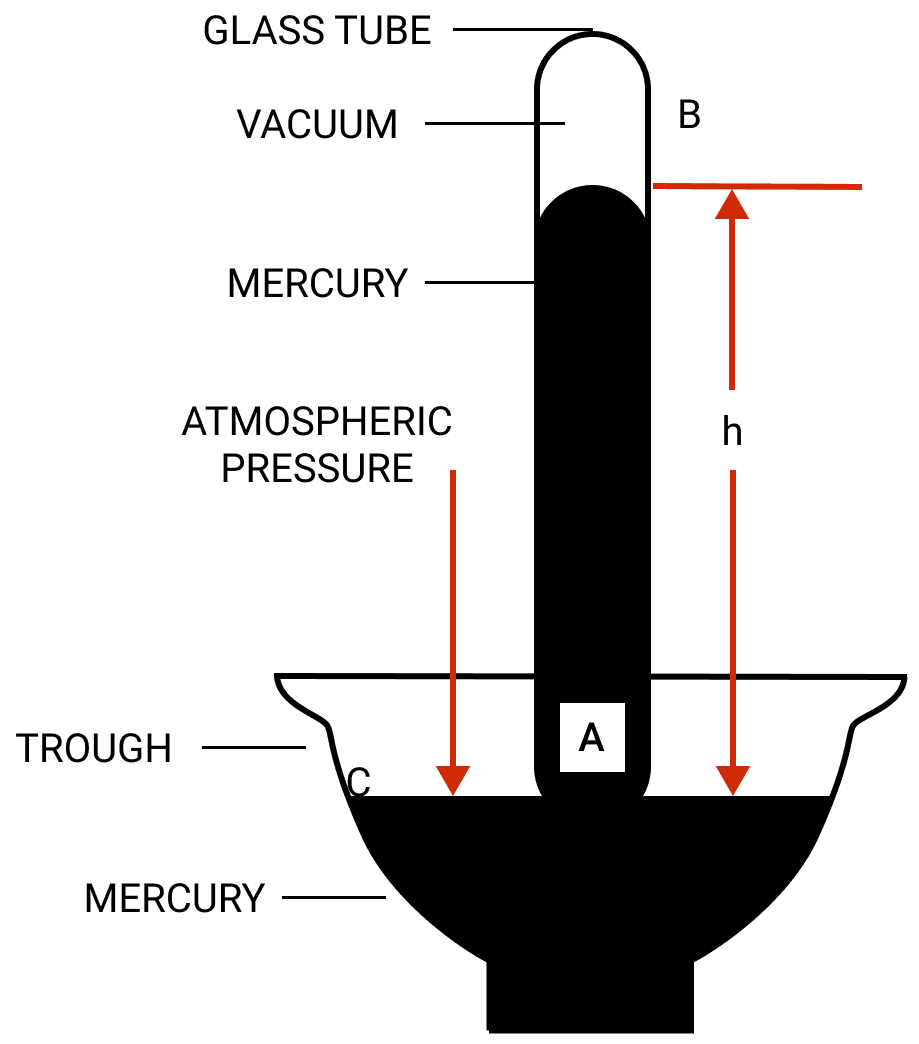
In figure, the atmospheric pressure acts at all points outside the tube (such as C) on the surface of mercury in the trough. The pressure at point A is due to the weight of the mercury column AB above it.
The mercury level in the tube becomes stationary when the pressure inside the tube at the point A which is at the level of the point C, becomes equal to that at the point C.
Thus, the vertical height of mercury column in it (i.e., AB = h) is a measure of atmospheric pressure and is called the barometric height.
The space left empty above the mercury column in the tube is called the torricellian vacuum. Ideally there should be no air in this space.
Answered By
21 Likes
Related Questions
Why does the liquid rise in a syringe when it's piston is pulled up ?
How is water drawn up from a well by a water pump?
Why is the barometric height used as a unit to express the atmospheric pressure ?
Illustrate with the help of a labelled diagram of a simple barometer that the atmospheric pressure at a place is 76 cm of Hg.