Chemistry
Draw the structural formula of carbon tetrachloride and state the type of bond present in it.
Chemical Bonding
195 Likes
Answer
Structural formula of carbon tetrachloride is given below:
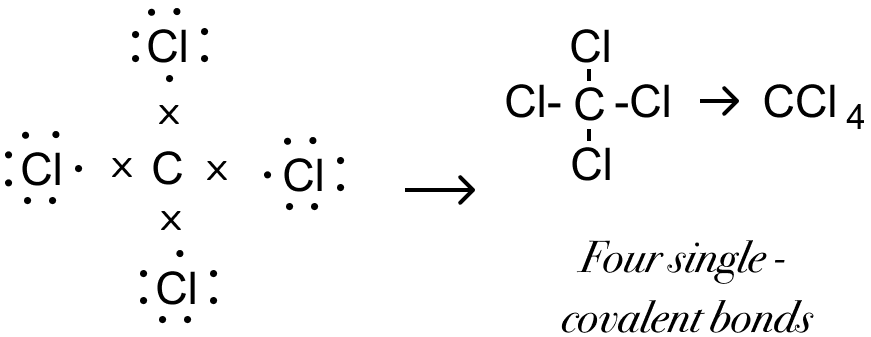
Covalent bond is present in carbon tetrachloride.
Answered By
130 Likes
Related Questions
State the terms defined in each case:
A bond formed by – (a) a shared pair of electrons, each bonding atom contributing one electron to the pair.
(b) a shared pair of electrons with both electrons coming from the same atom.
The one which is composed of all the three kinds of bond [ionic, covalent and coordinate bond] is:
- Sodium chloride
- Ammonia
- Carbon tetrachloride
- Ammonium chloride
Select the correct answer from A, B, C and D — Metals lose electrons during ionization — this change is called:
- Oxidation
- Reduction
- Redox
- Displacement
Select the right answer from the choices — covalent bond / ionic bond / covalent & coordinate bond for each of the following —
(i) Sodium chloride
(ii) Ammonium ion
(iii) Carbon tetrachloride