Chemistry
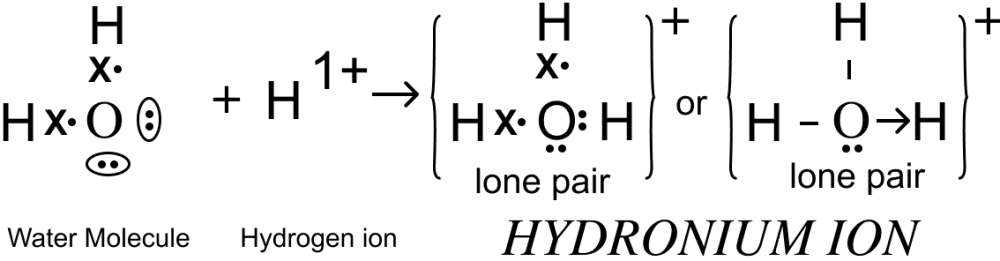
Draw an electron dot diagram of the structure of — hydronium ion. State the type of bonding present in it.
Related Questions
Draw an electron dot diagram: showing the lone pair effect for formation of — NH41+ ion from NH3 gas and H1+.
Give reasons — Hydrogen chloride can be termed as a polar covalent compound.
There are three elements E, F, G with atomic numbers 19, 8 and 17 respectively. Give the molecular formula of the compound formed between E and G and state the type of chemical bond in this compound.
A chemical term for: A bond formed by a shared pair of electrons with both electrons coming from the same atom.