Chemistry
Draw an electron dot diagram for the formation of each of the following :
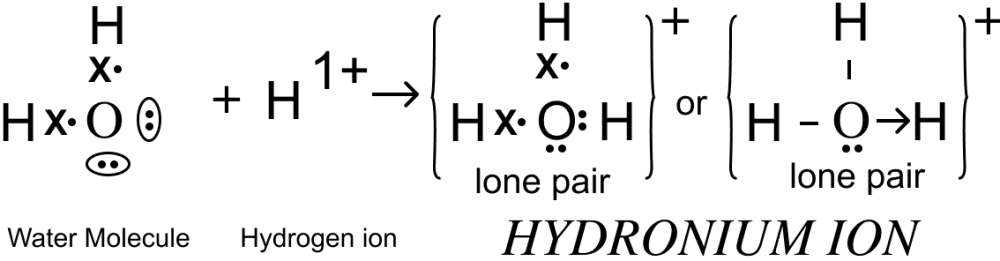
(i) Hydronium ion,
(ii) Ammonium ion,
(iii) Hydroxyl ion.
State the type of bonding present in them.
Related Questions
There are three elements E, F, G with atomic numbers 19, 8 and 17 respectively. Give the molecular formula of the compound formed between E and G and state the type of chemical bond in this compound.
Define a coordinate bond and give the conditions for its formation.
How many atoms of each kind are present in the following molecules : calcium oxide, chlorine, water, carbon tetrachloride ?
How many electrons are required or released by each atom mentioned in (a) to attain the nearest noble gas configuration ?