Physics
Answer
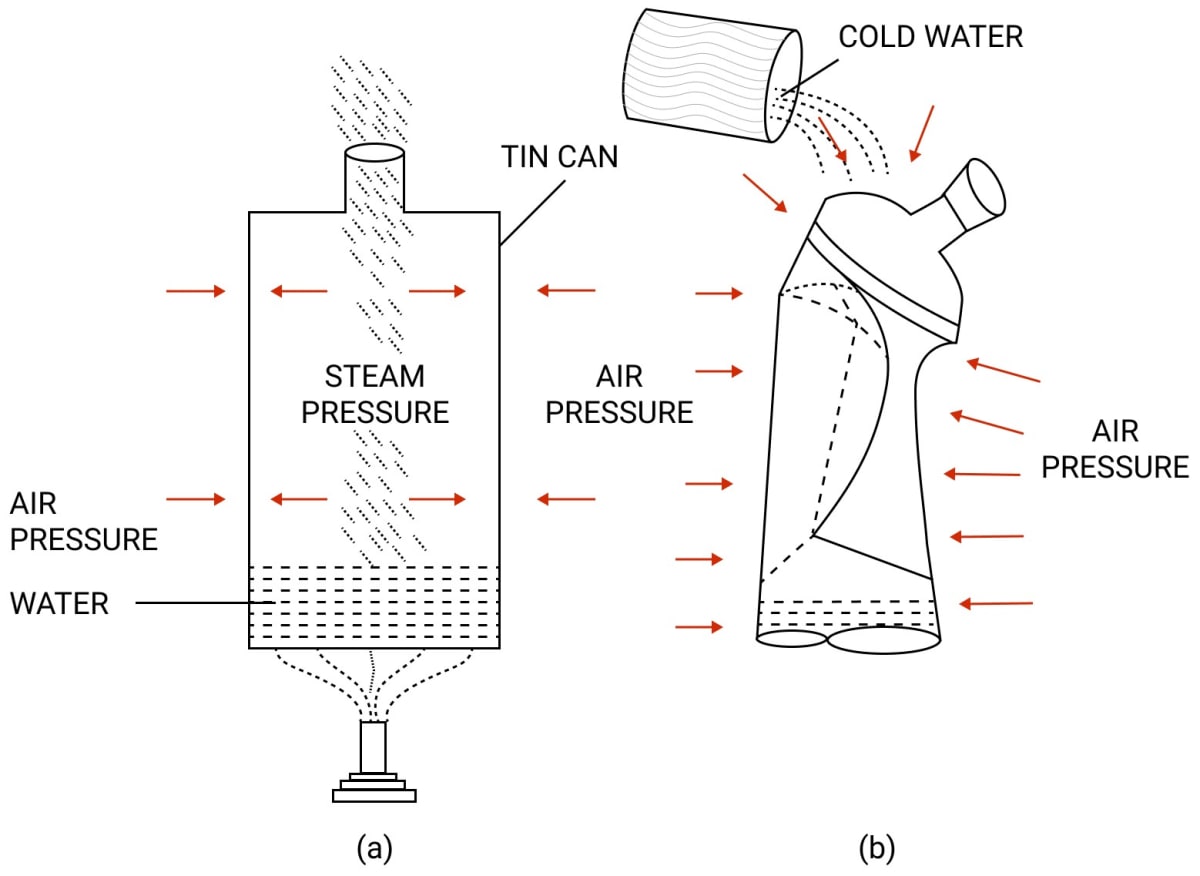
We take a thin tin can fitted with an airtight stopper. The stopper is removed and a small quantity of water is boiled in the can.
Gradually, the steam occupies the entire space of can by expelling the air from it, as shown in figure below. The stopper is then tightly replaced and simultaneously the flame beneath the can is removed. Cold water is then poured over the can. It is observed that the can collapses inward, as shown below.
The reason is that, initially the pressure due to steam inside the heated can is same as the air pressure outside the can.
But, on pouring cold water over the can, fitted with a stopper the steam inside the can condenses, producing water and water vapour at a very low pressure. Now the air pressure outside the can exceeds the vapour pressure inside the closed can.
Consequently, the excess atmospheric pressure outside causes it to collapse it inwards. This demonstrates that the atmosphere outside the can exerts a pressure which is known as atmospheric pressure.
Related Questions
Explain the following:
A balloon collapses when air is removed from it.
Explain the following:
Water does not run out of a dropper unless it's rubber bulb is pressed.
What do you understand by atmospheric pressure ?
We do not feel uneasy even under the enormous pressure of atmosphere above as well as around us. Give a reason.