Chemistry
At constant temperature, the effect of change of pressure on volume of a gas was as given below :
| Pressure in atmospheres | Volume in litres |
|---|---|
| 1.0 | 12 |
| 1.5 | 8.0 |
| 2.0 | 6.0 |
| 2.5 | 4.8 |
| 3.0 | 4.0 |
(a) Plot the following graphs :
(i) P vs V
(ii) P vs 1/V
(iii) PV vs P
(b) Assuming that the pressure values given above are correct, find the correct measurement of the volume at 2.3 atmosphere pressure.
Gas Laws
90 Likes
Answer
(a)
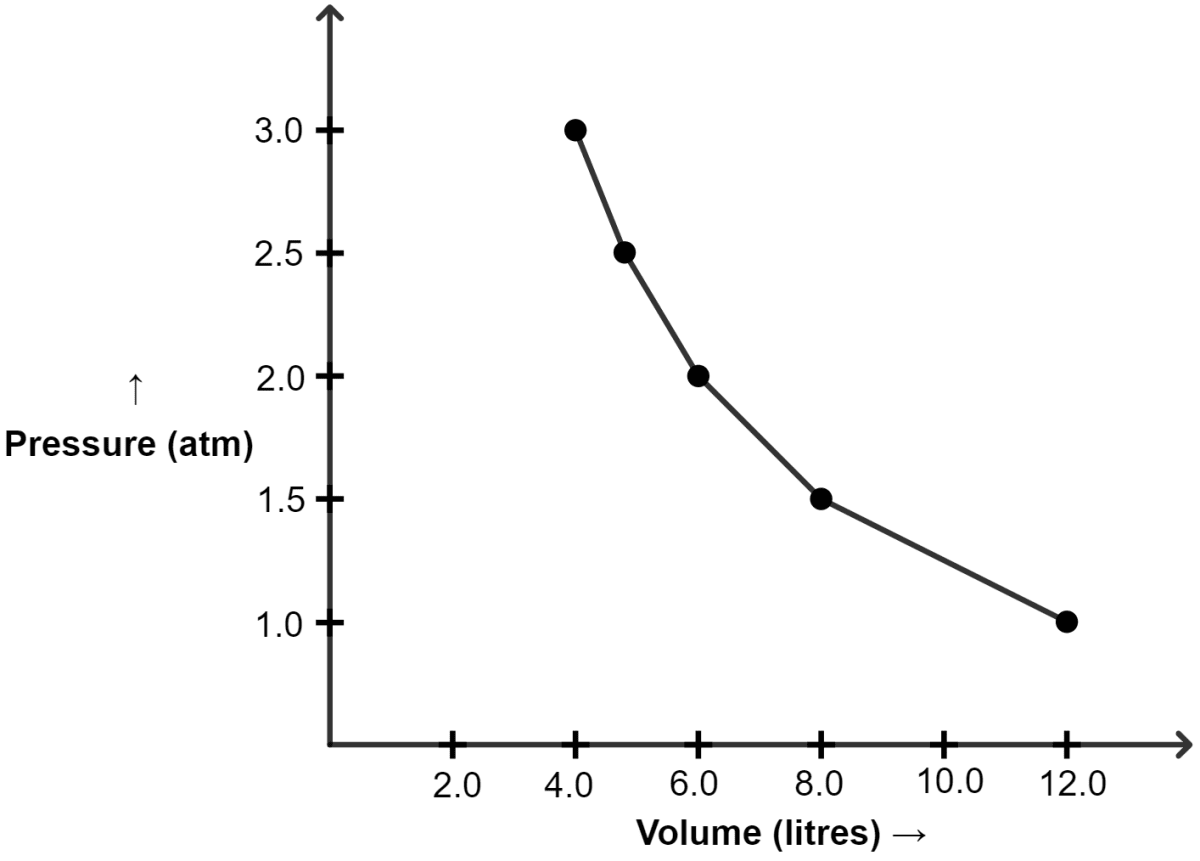
(i) Graph of P vs V :
| Pressure (atm) | Volume (lit) |
|---|---|
| 1.0 | 12.0 |
| 1.5 | 8.0 |
| 2.0 | 6.0 |
| 2.5 | 4.8 |
| 3.0 | 4.0 |
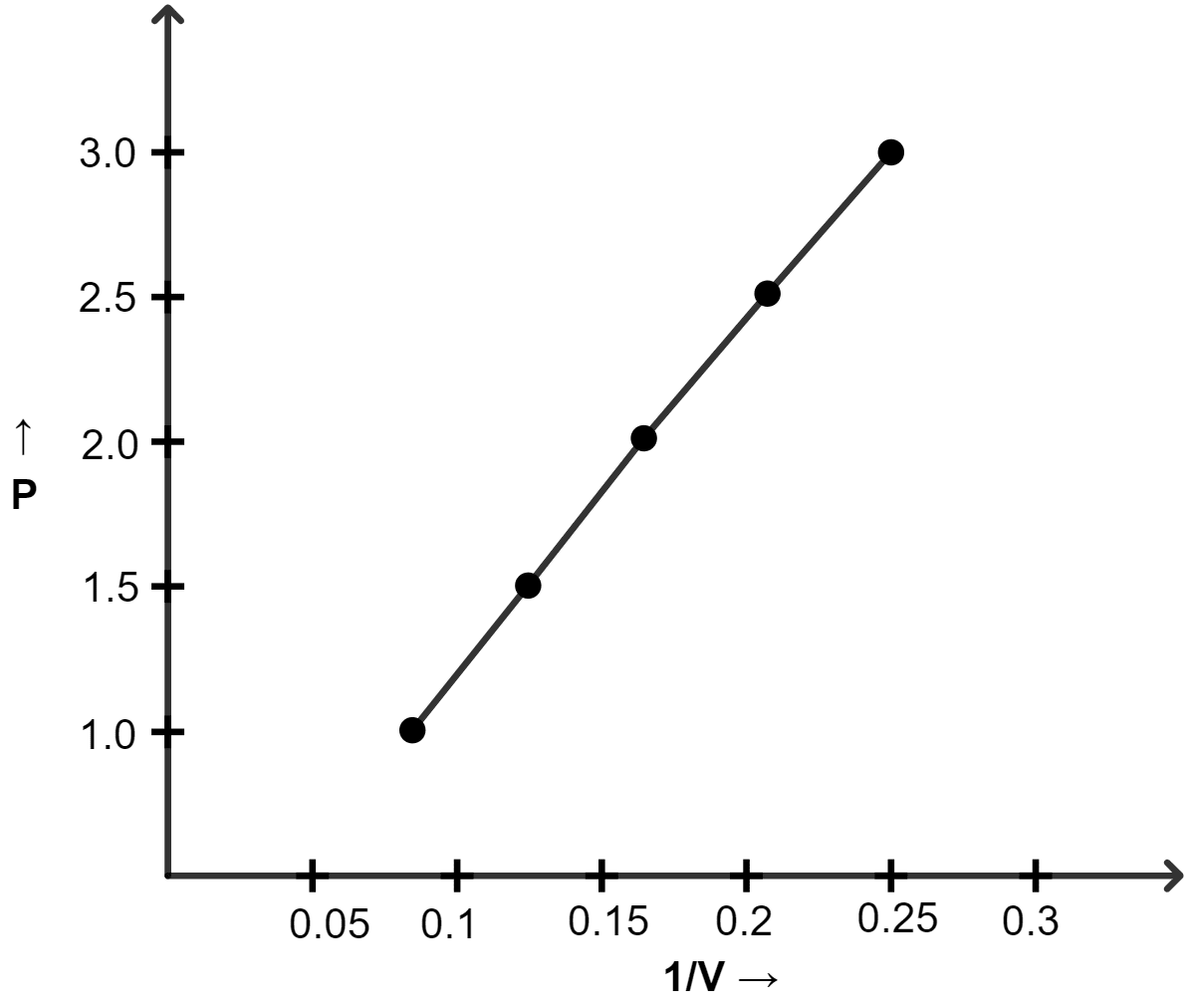
(ii) Graph of P vs 1/V :
| Pressure (atm) | Volume (lit) | 1/V |
|---|---|---|
| 1.0 | 12 | 0.083 |
| 1.5 | 8.0 | 0.125 |
| 2.0 | 6.0 | 0.166 |
| 2.5 | 4.8 | 0.208 |
| 3.0 | 4.0 | 0.25 |
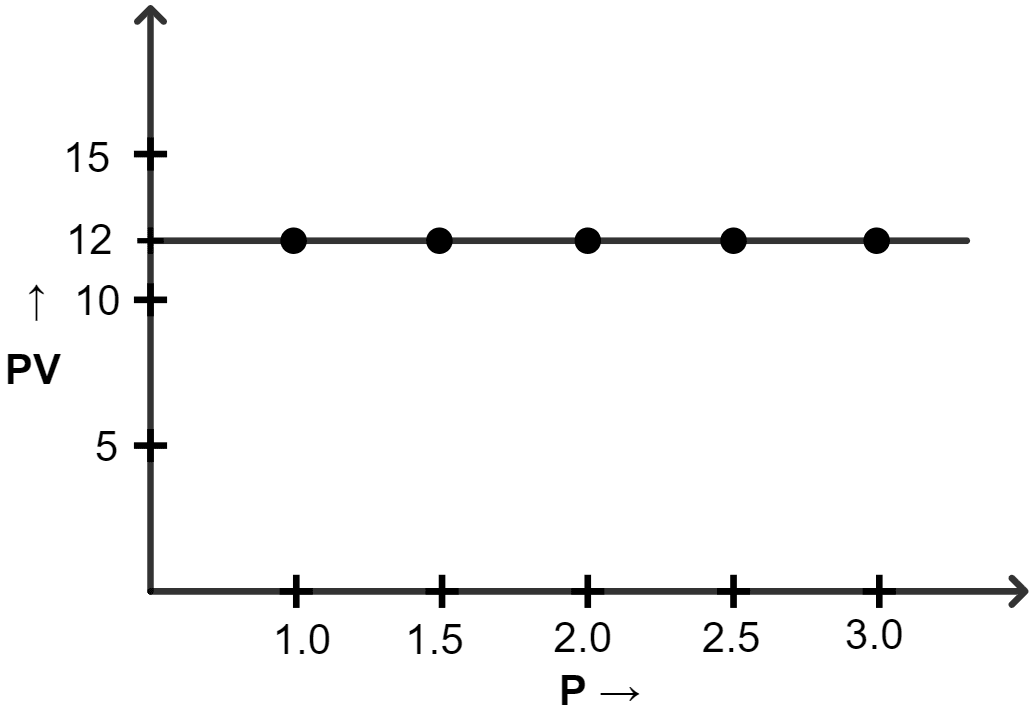
(iii) Graph of PV vs P :
| Pressure (atm) | Volume (lit) | PV |
|---|---|---|
| 1.0 | 12 | 12 |
| 1.5 | 8.0 | 12 |
| 2.0 | 6.0 | 12 |
| 2.5 | 4.8 | 12 |
| 3.0 | 4.0 | 12 |
(b) By Boyle's Law:
P1V1 = 12 is a constant, hence, V2 = ? at P2 = 2.3 atm
Substituting the values :
Hence, volume = 5.217 lit
Answered By
36 Likes
Related Questions
2 litres of a gas is enclosed in a vessel at a pressure of 760 mm Hg. If temperature remains constant, calculate pressure when volume changes to 4 dm3.
800 cm3 of gas is collected at 654 mm pressure. At what pressure would the volume of the gas reduce by 40% of its original volume, temperature remaining constant?
A cylinder of 20 litres capacity contains a gas at 100 atmospheric pressure. How many flasks of 200 cm3 capacity can be filled from it at 1 atmosphere pressure temperature remaining constant?
A steel cylinder of internal volume 20 litres is filled with hydrogen at 29 atmospheric pressure. If hydrogen is used to fill a balloon at 1.25 atmospheric pressure at the same temperature, what volume will the gas occupy ?