Physics
A certain mass of solid substance is heated for a period of 50 min. Its heating curve is shown below :

From the graph, which one of the following statement is correct?
- The boiling point of the given substance is 40°C.
- The specific latent heat of fusion of substance is the same as the specific latent heat of vaporisation.
- The substance has a higher specific heat capacity in solid state than that in liquid state.
- The substance has a lower specific heat capacity in solid state than that in liquid state.
Calorimetry
51 Likes
Answer
The substance has a higher specific heat capacity in solid state than that in liquid state
Reason — From graph, 40°C is the melting point of the substance. It takes 10 mins for melting for temperature change of 20°C (i.e.,40°-20°) and takes 10 mins for boiling for temperature change of 40°C (i.e., 80°-40°).
Hence, the latent heat of fusion is less than the latent heat of vapourisation
From relation,
Q = m x c x △T
C ∝
So, from graph, the specific heat capacity in solid state is greater than that in liquid state.
Answered By
19 Likes
Related Questions
The figure given below shows the cooling curve of pure wax material after heating. It cools from A and B and solidifies along BD. If L and c represents the value of latent heat and the specific heat of liquid wax, then the ratio of is
- 20
- 40
- 60
- 100

In the circuit shown, lamps L1 and L2, are identical. The sliding contact at O moved towards Q, then which of the statements about the effect on the brightness in L2 is correct?
- Initially it has no light, then its brightness increases gradually and becomes equal to that of L1
- Initially it is same as that of L1, and finally become half as that of L1
- Initially it is half as that of L1 and finally becomes equal to that of L1.
- Initially it is half as that of L1 and finally becomes zero.

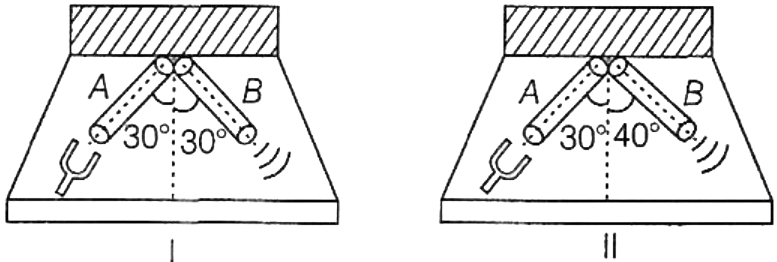
In which of the following figures shown, will the sound of a tuning fork hit with rubber pad at end A be heard maximum at end B?
- I
- II
- Both I and II
- Cannot be decided
The force of 20 N is applied at distance of 40 cm from the axis. The moment of force is
- 8 N-m
- 0.4 N-m
- 8.5 N-m
- 20 N-m