Physics
A cell of emf 1.8 V and internal resistance 2 Ω is connected in series with an ammeter of resistance 0.7 Ω and resistance of 4.5 Ω as shown in figure.
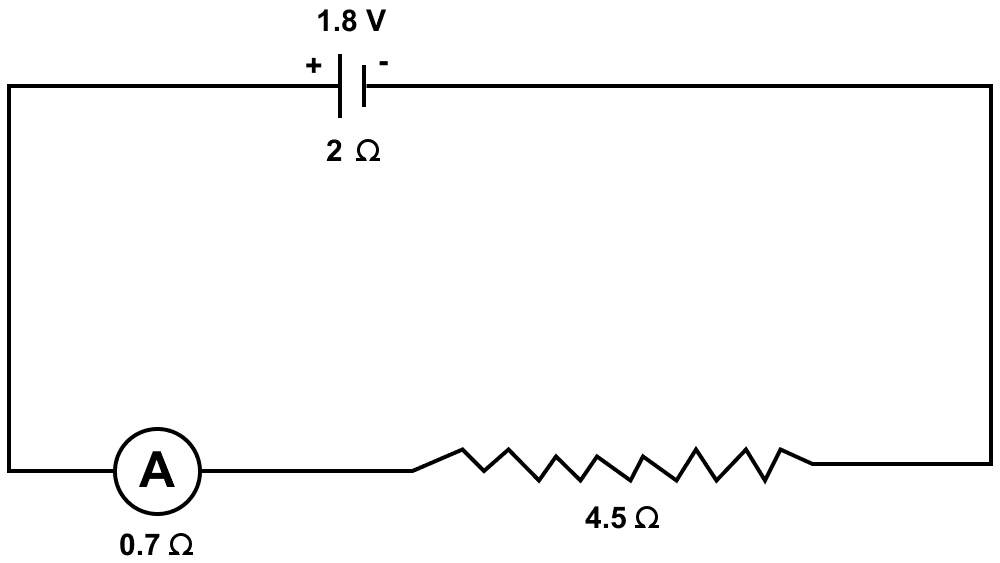
(a) What would be the reading of the ammeter?
(b) What is the potential difference across the terminals of the cell ?
Current Electricity
62 Likes
Answer
(a) Given,
e.m.f. (ε) = 1.8 V
Internal resistance (r) = 2 Ω
I = ?
Total resistance of arrangement = 2 + 0.7 + 4.5 = 7.2 Ω
From relation,
Substituting the values in the formula above we get,
Hence, reading of ammeter = 0.25 A
(b) Current (I) = 0.25 A
total resistance (excluding internal resistance) = 4.5 + 0.7 = 5.2 ohm
Using ohm's law
V = IR
Substituting the values in the formula above we get,
V = 0.25 × 5.2
V = 1.3 V
Hence, potential difference across the terminals of the battery = 1.3 V
Answered By
44 Likes
Related Questions
The diagram in figure shows a cell of e.m.f. ε = 2 volt and internal resistance r = 1 ohm connected to an external resistance R = 4 ohm. The ammeter A measures the current in the circuit and the voltmeter V measures the terminal voltage across the cell. What will be the readings of the ammeter and voltmeter when (i) the key K is open, and (ii) the key K is closed.

A battery of e.m.f. 6.0 V supplies current through a circuit in which resistance can be changed. A high resistance voltmeter is connected across the battery. When the current is 3 A, the voltmeter reads 5.4 V. Find the internal resistance of the battery.
A music system draws a current of 400 mA when connected to a 12 V battery.
(a) What is the resistance of the music system ?
(b) The music system if left playing for several hours and finally the battery voltage drops and the music system stops playing when the current drops to 320 mA. At what battery voltage does the music system stop playing.
A cell of e.m.f. ε and internal resistance r sends a current of 1.0 A when it is connected to an external resistance of 1.9 ohm. But it sends a current of 0.5 A when it is connected to an external resistance of 3.9 ohm. Calculate the values of ε and r.