Chemistry
(a) Ammonia reacts with excess chorine to form …………… (nitrogen / nitrogen trichloride / ammonium chloride).
(b) Give a balanced equation: Ammonium hydroxide is added to ferrous sulphate solution.
(c) Write observations: Ammonia gas is passed over heated copper (II) oxide.
Ammonia
15 Likes
Answer
(a) Ammonia reacts with excess chlorine to form nitrogen trichloride
NH3 + 3Cl2 [excess] ⟶ 3HCl + NCl3
(b) FeSO4 + 2NH4OH ⟶ (NH4)2SO4 + Fe(OH)2 ↓
(c) Black copper [II] oxide is reduced to brown copper.
2NH3 + 3CuO ⟶ 3Cu + 3H2O + N2 [g]
Answered By
11 Likes
Related Questions
The following questions are based on the preparation of ammonia gas in the laboratory:
(i) Explain why ammonium nitrate is not used in the preparation of ammonia.
(ii) Name the compound normally used as a drying agent during the process.
(iii) How is ammonia gas collected? Explain why it is not collected over water.
The diagram shows set up for the lab. preparation of a pungent alkaline gas.

(i) Name the gas collected in the jar.
(ii) Give a balanced equation for the above preparation
(iii) State how the above gas is collected?
(iv) Name the drying agent used.
(v) State how you will find out that the jar is full of the pungent gas?
Answer the following questions with respect to the given figure.
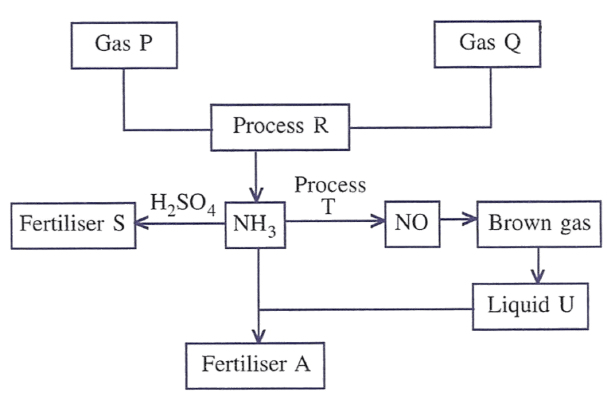
(a) Identify gas P and gas Q.
(b) Give a balanced equation to convert ammonia into gas P by a method other than decomposition. State the property of ammonia used in carrying out the conversion.
(c) Name fertiliser S and give a balanced equation for its preparation.
(d) Name process T and state the conditions that enable conversion of ammonia to nitric oxide.
(e) Give a balanced equation for the conversion of brown gas to liquid U.
(f) Name fertiliser A.
(g) Name process R.
The diagram given below describes the manufacturing process of a gas.
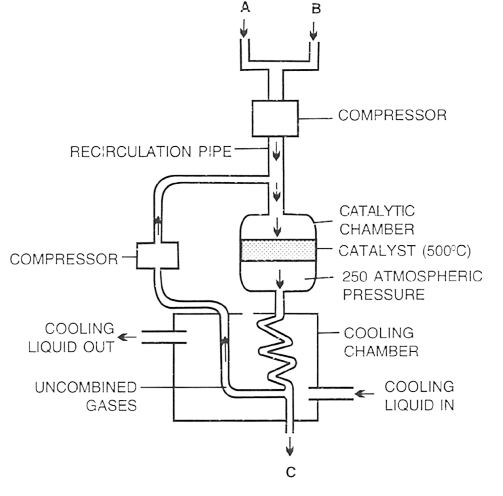
(a) Name the process.
(b) Identify A, B and C.
(c) State the ratio of A and B
(d) Write the equation involved with their respective conditions.
(e) How is the product separated from unreacted reactants ?