The salt solution which does not react with ammonium hydroxide is:
- Calcium Nitrate
- Zinc Nitrate
- Lead Nitrate
- Copper Nitrate
Answer
Calcium Nitrate
Reason — No ppt. occurs even with addition of excess of ammonium hydroxide as the concentration of OH- ions from the ionization of of NH4OH is so low that it cannot precipitate the hydroxide of calcium.
The organic compound which undergoes substitution reaction is:
- C2H2
- C2H4
- C10H18
- C2H6
Answer
C2H6
Reason — C2H6 will undergo substitution reaction as it is a saturated hydrocarbon and substitution reaction is a characteristic of saturated hydrocarbons.
The electrolysis of acidified water is an example of:
- Reduction
- Oxidation
- Redox reaction
- Synthesis
Answer
Redox reaction
Reason — At cathode, H+ ions gain electron and become neutral hydrogen atoms. Therefore, reduction takes place at cathode.
At anode, OH- ions lose electron and becomes neutral OH atoms. Therefore, oxidation takes place at anode.
Hence, electrolysis of acidified water is an example of redox reaction.
The IUPAC name of dimethyl ether is:
- Ethoxy methane
- Methoxy methane
- Methoxy ethane
- Ethoxy ethane
Answer
Methoxy methane
Reason — The IUPAC name of dimethyl ether is Methoxy methane.
The catalyst used in the Contact Process is:
- Copper
- Iron
- Vanadium pentoxide
- Manganese dioxide
Answer
Vanadium pentoxide
Reason — The catalyst used in the Contact Process is Vanadium pentoxide.
Give one word or a phrase for the following statements:
(i) The energy released when an electron is added to a neutral gaseous isolated atom to form a negatively charged ion.
(ii) Process of formation of ions from molecules which are not in ionic state.
(iii) The tendency of an element to form chains of identical atoms.
(iv) The property by which certain hydrated salts, when left exposed to atmosphere, lose their water of crystallization and crumble into powder.
(v) The process by which sulphide ore is concentrated.
Answer
(i) Electron affinity
(ii) Ionization
(iii) Catenation
(iv) Efflorescence
(v) Froth flotation process
Write a balanced chemical equation for each of the following:
(i) Action of concentrated sulphuric acid on carbon.
(ii) Reaction of sodium hydroxide solution with iron (III) chloride solution.
(iii) Action of heat on aluminum hydroxide.
(iv) Reaction of zinc with potassium hydroxide solution.
(v) Action of dilute hydrochloric acid on magnesium sulphite.
Answer
(i) C + 2H2SO4 [conc.] ⟶ CO2 + 2H2O + 2SO2
(ii) FeCl3 + 3NaOH ⟶ 3NaCl + Fe(OH)3
(iii) 2Al(OH)3 Al2O3 + 3H2O
(iv) Zn + 2KOH ⟶ K2ZnO2 + H2
(v) MgSO3 + 2HCl ⟶ MgCl2 + H2O + SO2 [g]
(i) Give the IUPAC name for each of the following:
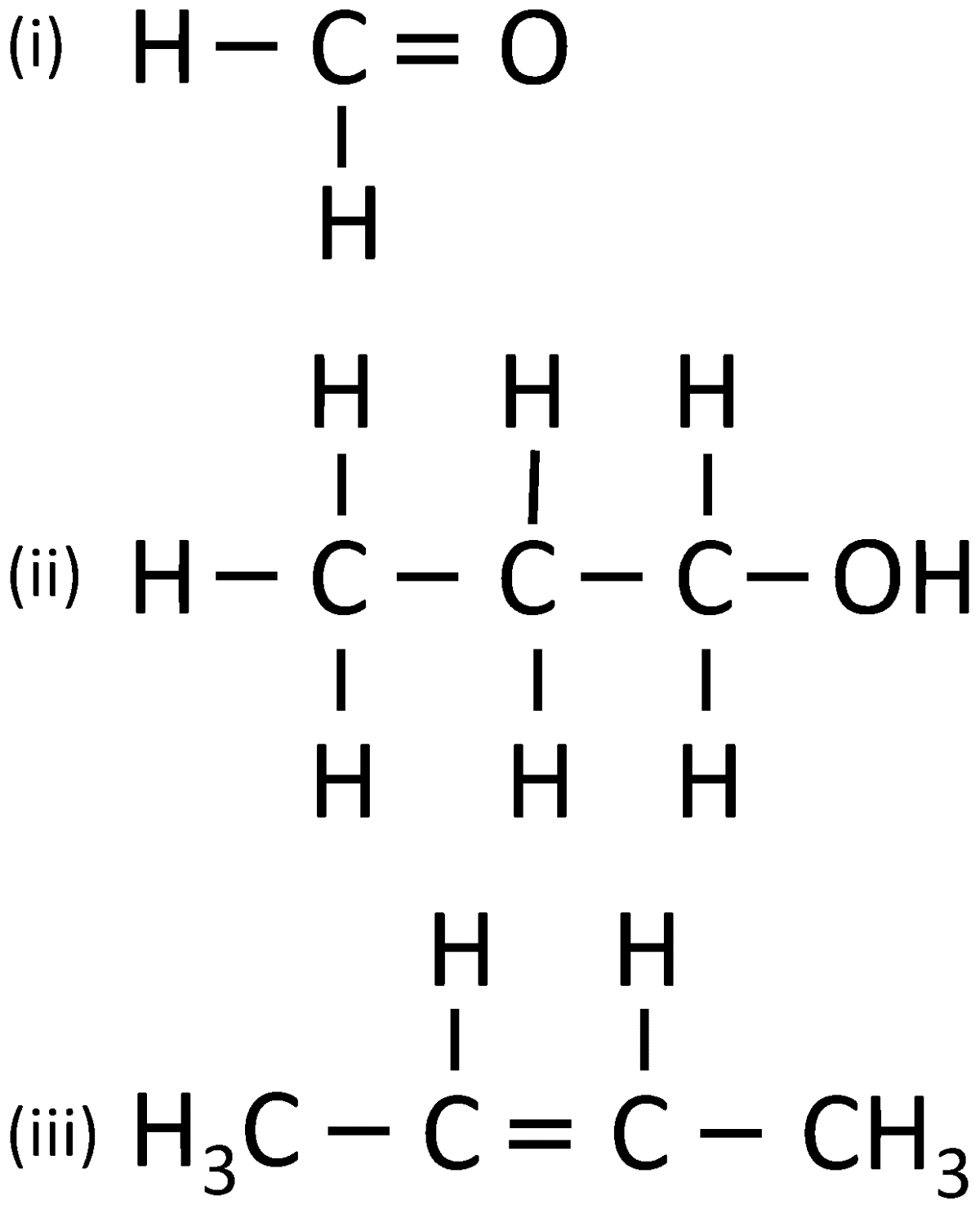
(ii) Write the structural formula of the two isomers of butane.
Answer
- Methanal
- 1-Propanol
- 2-Butene
(ii) Structural formula of the two isomers of butane are given below:
- Butane [n-butane]

- 2-Methyl propane [iso-butane]

State one relevant observation for each of the following:
(i) Lead nitrate solution is treated with sodium hydroxide solution drop wise till it is in excess.
(ii) At the anode, when molten lead bromide is electrolyzed using graphite electrodes.
(iii) Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
(iv) Anhydrous calcium chloride is exposed to air for some time.
(v) Barium chloride solution is slowly added to sodium sulphate solution.
Answer
(i) Chalky white precipitate is formed which is soluble in excess of sodium hydroxide and forms a colourless solution.
(ii) At the anode, brown fumes of bromine vapours are observed.
(iii) White precipitate of PbCl2 is formed which is soluble in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 + 2HNO3
(iv) It absorbs moisture from the atmosphere, dissolve in the same and change into a solution. This property is known as deliquescence
(v) A white ppt. is obtained which is insoluble in dil. HCl or dil. HNO3
Na2SO4 + BaCl2 ⟶ BaSO4 ↓ [white ppt.] + 2NaCl
Give a reason for each of the following:
(i) Ionic compounds have a high melting point.
(ii) Inert gases do not form ions.
(iii) Ionisation potential increases across a period, from left to right.
(iv) Alkali metals are good reducing agents.
(v) Conductivity of dilute hydrochloric acid is greater than that of acetic acid.
Answer
(i) Ionic compounds have ionic bonds so there exists a strong force of attraction between the oppositely charged ions, so a large amount of energy is required to break the strong bonding between the ions. Hence, ionic compounds have a high melting point.
(ii) Inert gases have completely filled octet which makes them extremely stable. They neither lose, nor gain electrons. Hence, they do not form ions.
(iii) The ionisation potential of an element increases across a period because the atomic size decreases due to an increase in nuclear charge and electrons in the outermost shell are more strongly held because of which greater energy is required to remove the electron.
(iv) Alkali metals have one electron in the outermost shell and hence during reactions they readily give the valence electron and get themselves oxidised. Hence, they are good reducing agents.
(v) Dil. H2SO4 is a strong electrolyte and acetic acid is a weak electrolyte. Therefore, dil. H2SO4 allows large amount of electricity to flow through it and is a good conductor of electricity whereas acetic acid allows small amount of electricity to flow through it and is a poor conductor of electricity. Hence, electrical conductivity of dilute hydrochloric acid is greater than that of acetic acid.
Name the gas that is produced in each of the following cases:
(i) Sulphur is oxidized by concentrated nitric acid.
(ii) Action of dilute hydrochloric acid on sodium sulphide.
(iii) Action of cold and dilute nitric acid on copper.
(iv) At the anode during the electrolysis of acidified water.
(v) Reaction of ethanol and sodium.
Answer
(i) Nitrogen dioxide gas
(ii) Hydrogen sulphide gas
(iii) Nitric oxide gas
(iv) Oxygen gas
(v) Hydrogen gas
Fill up the blanks with the correct choice given in brackets.
(i) Ionic or electrovalent compounds do not conduct electricity in their ............... state. (fused / solid)
(ii) Electrolysis of aqueous sodium chloride solution will form ............... at the cathode. (hydrogen gas / sodium metal)
(iii) Dry hydrogen chloride gas can be collected by............... displacement of air. (downward / upward)
(iv) The most common ore of iron is ............... (calamine / haematite)
(v) The salt prepared by the method of direct combination is ............... (iron (II) chloride / iron (III) chloride)
Answer
(i) Ionic or electrovalent compounds do not conduct electricity in their solid state.
(ii) Electrolysis of aqueous sodium chloride solution will form hydrogen gas at the cathode.
(iii) Dry hydrogen chloride gas can be collected by upward displacement of air.
(iv) The most common ore of iron is haematite
(v) The salt prepared by the method of direct combination is iron (III) chloride.
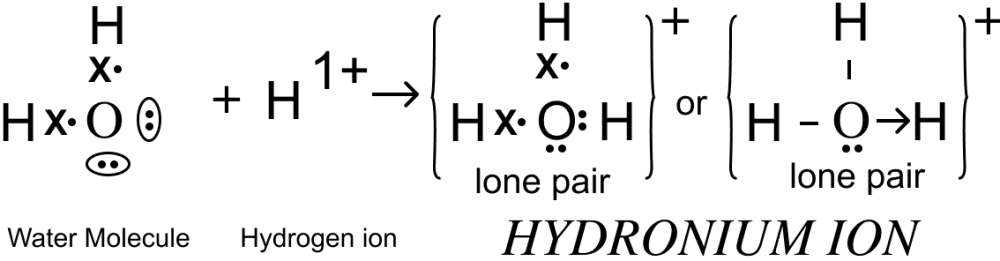
(i) What do you understand by a lone pair of electrons?
(ii) Draw the electron dot diagram of Hydronium ion. (H=1; O=8)
Answer
(i) Lone pair of electrons are valence electrons that in a covalent bond are not shared with another atom.
(ii) Electron dot diagram of Hydronium ion is shown below:
In Period 3 of the Periodic Table, element B is placed to the left of element A.
On the basis of this information, choose the correct word from the brackets to complete the following statements:
(i) The element B would have (lower / higher) metallic character than A.
(ii) The element A would probably have (lesser / higher) electron affinity than B.
(iii) The element A would have (greater / smaller) atomic size than B.
Answer
If the element are placed as B and then A in the 3rd period of the periodic table then
(i) The element B would have higher metallic character than A as metallic character decreases across a period.
(ii) The element A would probably have higher electron affinity than B as electron affinity increases across a period.
(iii) The element A would have smaller atomic size than B as atomic size decreases across a period.
Copy and complete the following table which refers to the conversion of ions to neutral particles.
| Conversion | Ionic equation | Oxidation/Reduction |
|---|---|---|
| Chloride ion to chlorine molecule | ||
| Lead [II] ion to lead |
Answer
| Conversion | Ionic equation | Oxidation/Reduction |
|---|---|---|
| Chloride ion to chlorine molecule | Cl1- - 1e- ⟶ Cl [Cl2] Cl + Cl ⟶ Cl2 | Oxidation |
| Lead [II] ion to lead | Pb2+ + 2e- ⟶ Pb | Reduction |
(i) Write the balanced chemical equation to prepare ammonia gas in the laboratory by using an alkali.
(ii) State why concentrated sulphuric acid is not used for drying ammonia gas.
(iii) Why is ammonia gas not collected over water?
Answer
(i) 2NH4Cl + Ca(OH)2 ⟶ CaCl2 + 2H2O + 2NH3 [g]
(ii) As sulphuric acid reacts chemically with ammonia to form ammonium sulphate hence, it is not used as a drying agent for drying ammonia.
2NH3 + H2SO4 ⟶ (NH4)2SO4
(iii) As ammonia gas is highly soluble in water hence, it is not collected over water.
(i) Name the acid used for the preparation of hydrogen chloride gas in the laboratory. Why is this particular acid preferred to other acids?
(ii) Write the balanced chemical equation for the laboratory preparation of hydrogen chloride gas.
Answer
(i) Conc. H2SO4 is used for the preparation of hydrogen chloride gas in the laboratory.
As conc. H2SO4 is non-volatile and has a high boiling point, therefore, it displaces the volatile hydrogen chloride from the salt sodium chloride. Hence, conc. H2SO4 is used as a reactant in the laboratory preparation of HCl from sodium chloride.
(ii)
For the preparation of hydrochloric acid in the laboratory:
(i) Why is direct absorption of hydrogen chloride gas in water not feasible?
(ii) What arrangement is done to dissolve hydrogen chloride gas in water?
Answer
(i) Hydrogen chloride gas is sufficiently soluble, so, it is absorbed more quickly than it is being generated in the flask. This causes back-suction due to partial vacuum created in the tube. Hence, direct absorption of hydrogen chloride gas in water is not feasible.
(ii) A special funnel arrangement is done in which an inverted funnel, connected to the hydrogen chloride gas supply is placed in the beaker in such a way that it just touches the water taken in the trough. This arrangement minimizes back suction.
For the electro-refining of copper:
(i) What is the cathode made up of?
(ii) Write the reaction that takes place at the anode.
Answer
(i) Pure thin sheet of copper.
(ii) Cu - 2e- ⟶ Cu2+
The percentage composition of a gas is:
Nitrogen 82.35%, Hydrogen 17.64%.
Find the empirical formula of the gas. [N = 14, H = 1]
Answer
| Element | % composition | At. wt. | Relative no. of atoms | Simplest ratio |
|---|---|---|---|---|
| Nitrogen | 82.35 | 14 | = 5.88 | = 1 |
| Hydrogen | 17.64 | 1 | = 17.64 | = 3 |
Simplest ratio of whole numbers = N : H = 1 : 3
Hence, empirical formula is NH3
Aluminum carbide reacts with water according to the following equation:
Al4C3 + 12H2O ⟶ 4Al(OH)3 + 3CH4
(i) What mass of aluminum hydroxide is formed from 12g of aluminum carbide?
(ii) What volume of methane at s.t.p. is obtained from 12g of aluminum carbide?
[Relative molecular weight of Al4C3 = 144; Al(OH)3 = 78]
Answer
144 g of aluminium carbide forms 312 g of aluminium hydroxide.
∴ 12 g of aluminium carbide will form x 12 = 26 g of aluminium hydroxide
Hence, 26 g of aluminium hydroxide is formed.
(ii) 144 g of aluminium carbide forms 67.2 lit of methane.
∴ 12 g of aluminium carbide will form x 12 = 5.6 lit.
Hence, vol. of methane obtained at s.t.p. = 5.6 lit.
(i) If 150 cc of gas A contains X molecules, how many molecules of gas B will be present in 75 cc of B?
The gases A and B are under the same conditions of temperature and pressure.
(ii) Name the law on which the above problem is based.
Answer
(i) According to Avogadro’s law : equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.
Given,
150 cc of a gas A contains X molecules
Therefore, 150 cc of B will also contain X molecule.
Hence, 75 cc of B will contain X/2 molecules.
(ii) The problem is based on Avogadro's law.
Name the main component of the following alloys:
(i) Brass
(ii) Duralumin
Answer
(i) Copper
(ii) Aluminium
Complete the following table which relates to the homologous series of hydrocarbons.
| General formula | IUPAC name of the homologous series | Characteristic bond type | IUPAC name of the first member of the series |
|---|---|---|---|
| CnH2n-2 | (A) ............... | (B) ............... | (C)............... |
| CnH2n+2 | (D)............... | (E) ............... | (F)............... |
Answer
| General formula | IUPAC name of the homologous series | Characteristic bond type | IUPAC name of the first member of the series |
|---|---|---|---|
| CnH2n-2 | (A) Alkynes | (B) Triple covalent bond | (C) Ethyne |
| CnH2n+2 | (D) Alkanes | (E) Single covalent bond | (F) Methane |
(i) Name the most common ore of the metal aluminum from which the metal
is extracted. Write the chemical formula of the ore.
(ii) Name the process by which impure ore of aluminum gets purified by using concentrated solution of an alkali.
(iii) Write the equation for the formation of aluminum at the cathode during the electrolysis of alumina.
Answer
(i) Bauxite [Al2O3.2H2O]
(ii) Bayer's Process
(iii) 2Al3+ + 6e- ⟶ 2Al
A compound X (having vinegar like smell) when treated with ethanol in the presence of the acid Z, gives a compound Y which has a fruity smell.
The reaction is:
(i) Identify Y and Z.
(ii) Write the structural formula of X.
(iii) Name the above reaction.
Answer
(i) Y is ethyl acetate and Z is conc. sulphuric acid
(ii) Structural formula of X (ethanoic acid) is shown below:

(iii) Esterification reaction
Ethane burns in oxygen to form CO2 and H2O according to the equation:
2C2H6 + 7O2 ⟶ 4CO2 + 6H2O.
If 1250 cc of oxygen is burnt with 300 cc of ethane.
Calculate:
(i) the volume of CO2 formed.
(ii) the volume of unused O2.
Answer
[By Lussac's law]
(i) To calculate the volume of CO2 formed :
∴
Hence, volume of carbon dioxide formed = 600 cc
(ii) To calculate the volume of unused O2 :
∴
Unused oxygen = 1250 - 1050 = 200 cc.
Hence, volume of unused oxygen = 200 cc
Three solutions P, Q and R have pH value of 3.5, 5.2 and 12.2 respectively.
Which one of these is a:
(i) Weak acid?
(ii) Strong alkali?
Answer
(i) Q
Reason — On a pH scale, acids have pH less than 7 whereas weak acids have pH towards 7. Hence, Q will be a weak acid with pH 5.2 .
(ii) R
Reason — On a pH scale, alkali have pH more than 7 and alkalinity increases as the pH value moves away from 7. Hence, R will be a strong alkali with pH 12.2 .
Give a chemical test to distinguish between the following pairs of chemicals:
(i) Lead nitrate solution and Zinc nitrate solution
(ii) Sodium chloride solution and Sodium nitrate solution
Answer
(i) When NaOH is added to each of the compounds, lead nitrate forms a chalky white precipitate of lead hydroxide [Pb(OH)2] whereas a gelatinous white precipitate of zinc hydroxide [Zn(OH)2] is formed in case of zinc nitrate.
(ii) Add silver nitrate soln. to the given solns., sodium chloride reacts to form a white ppt. which is soluble in NH4OH and insoluble in dil. HNO3. The other soln. is sodium nitrate.
NaCl + AgNO3 ⟶ AgCl + NaNO3
NaNO3 + AgNO3 ⟶ no white ppt.
Write a balanced equation for the preparation of each of the following salts:
(i) Copper sulphate from Copper carbonate.
(ii) Zinc carbonate from Zinc sulphate.
Answer
(i) CuCO3 + H2SO4 ⟶ CuSO4 + H2O + CO2
(ii) ZnSO4 + (NH4)2CO3 ⟶ (NH4)2SO4 + ZnCO3
(i) What is the type of salt formed when the reactants are heated at a suitable temperature for the preparation of Nitric acid?
(ii) State why for the preparation of Nitric acid, the complete apparatus is made up of glass.
Answer
(i) Acid salt
(ii) The vapours of nitric acid are highly corrosive and attack rubber, cork. etc. For this reason, all glass apparatus is used in the laboratory preparation of nitric acid.
Which property of sulphuric acid is shown by the reaction of concentrated sulphuric acid with:
(i) Ethanol?
(ii) Carbon?
Answer
(i) Dehydrating property
(ii) Oxidizing property
C + 2H2SO4 (conc.) ⟶ CO2 + 2SO2 + 2H2O