| ACIDS — Ions present in acids | Complete and balance the equations |
|---|---|
| a. Definition — Dissolves in water yielding hydronium ions | |
| 1. Hydrochloric acid | HCl ⇌ ............... + Cl- [H+ + H2O ⇌ ............... ] HCl + H2O ⇌ ............... + ............... |
| 2. Nitric acid | HNO3 + H2O ⇌ ............... + ............... |
| 3. Sulphuric acid | H2SO4 + H2O ⇌ ............... + ............... |
| b. Classification | |
| 4. Monobasic acid [Basicity = 1 ] | HCl [aq.] ⇌ ............... + ............... |
| 5. Dibasic acid [Basicity = 2] | H2SO4 [aq.] ⇌ ............... + ............... |
| 6. Tribasic acid [Basicity = 3] | H3PO4 [aq.] ⇌ ............... + ............... |
| c. Preparation of acids | |
| 7. From non-metals | H2 + Cl2 ⟶ ............... |
| 8. From acidic oxides | CO2 + H2O ⟶ ............... |
| SO3 + H2O ⟶ ............... | |
| P2O5 + H2O ⟶ ............... | |
| 9. From normal salts | KNO3 + H2SO4 ⟶ ............... + ............... |
| NaCl+ H2SO4 ⟶ ............... + ............... | |
| 10. By oxidation of non-metals | S + HNO3 ⟶ ............... + H2O ............... [g] |
| d. Properties of acids | |
| 11. Neutralizes bases | CuO + H2SO4 ⟶ ............... + ............... |
| NaOH + HCl ⟶ ............... + ............... | |
| Reaction with | |
| 12. Chlorides and nitrates | |
| 13. Carbonates and bicarbonates | Na2CO3 + H2SO4 ⟶ ............... + H2O + ............... [g] |
| NaHCO3 + H2SO4 ⟶ ............... + H2O + ............... [g] | |
| 14. Sulphites and bisulphites | Na2SO3 + HCl ⟶ ............... + H2O + ............... [g] |
| NaHSO3 + HCl ⟶ ............... + H2O + ............... [g] | |
| 15. Active metals | Zn + HCl ⟶ ............... + ............... [g] |
Answer
| ACIDS — Ions present in acids | Complete and balance the equations |
|---|---|
| a. Definition — Dissolves in water yielding hydronium ions | |
| 1. Hydrochloric acid | HCl ⇌ H+ + Cl- [H+ + H2O ⇌ H3O+] HCl + H2O ⇌ H3O+ + Cl- |
| 2. Nitric acid | HNO3 + H2O ⇌ H3O+ + NO3- |
| 3. Sulphuric acid | H2SO4 + 2H2O ⇌ 2H3O+ + SO42- |
| b. Classification | |
| 4. Monobasic acid [Basicity = 1 ] | HCl [aq.] ⇌ H3O+ + Cl- |
| 5. Dibasic acid [Basicity = 2] | H2SO4 [aq.] ⇌ 2H3O+ + SO42- |
| 6. Tribasic acid [Basicity = 3] | H2PO4 [aq.] ⇌ 3H3O+ + PO43- |
| c. Preparation of acids | |
| 7. From non-metals | H2 + Cl2 ⟶ 2HCl |
| 8. From acidic oxides | CO2 + H2O ⟶ H2CO3 |
| SO3 + H2O ⟶H2SO4 | |
| P2O5 + 3H2O ⟶2H3PO4 | |
| 9. From normal salts | KNO3 + H2SO4 ⟶KHSO4 + HNO3 |
| NaCl+ H2SO4 ⟶NaHSO4 + HCl | |
| 10. By oxidation of non-metals | S + 6HNO3 ⟶H2SO4 + 2H2O + 6NO2 [g] |
| d. Properties of acids | |
| 11. Neutralizes base | CuO + H2SO4 ⟶ CuSO4 + H2O |
| NaOH + HCl ⟶ NaCl + H2O | |
| Reaction with | |
| 12. Chlorides and nitrates | |
| 13. Carbonates and bicarbonates | Na2CO3 + H2SO4 ⟶ Na2SO4 + H2O + CO2 [g] |
| 2NaHCO3 + H2SO4 ⟶ Na2SO4 + 2H2O + 2CO2 [g] | |
| 14. Sulphites and bisulphites | Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2 [g] |
| NaHSO3 + HCl ⟶ NaCl + H2O + SO2 [g] | |
| 15. Active metals | Zn + 2HCl ⟶ ZnCl2 + H2 [g] |
| BASES — Ions present in bases | |
|---|---|
| a. Alkali — dissociates yielding hydroxyl ions | |
| 16. Sodium hydroxide | NaOH [aq.] ⇌ ............... + ............... |
| 17. Ammonium hydroxide | NH4OH [aq.] ⇌ ............... + ............... |
| b. Classification | |
| 18. Monoacidic base [Acidity = 1 ] | KOH [aq.] ⇌ ............... + ............... |
| 19. Diacidic base [Acidity = 2] | Cu(OH)2 [aq.] ⇌ ............... + ............... |
| c. Preparation of bases | |
| 20. From metals | Na + O2 ⟶ ............... |
| 21. From metallic oxides and metals | K2O + H2O ⟶ ............... |
| K + H2O ⟶ ............... + ............... | |
| 22.From salts | AlCl3 + NaOH ⟶ ............... + ............... ↓ |
| FeSO4 + NaOH ⟶ ............... + ............... ↓ | |
| 23. By thermal decomposition | ZnCO3 ⟶ ............... + ............... [g] |
| Pb(NO3)2 ⟶ ............... + ...............[g] + .............. [g] | |
| c. Properties of bases | |
| 24. Neutralizes acids | PbO + HNO3 ⟶ ............... + ............... |
| Fe(OH)2 + HCl ⟶ ............... + ............... | |
| Reaction with | |
| 25. Metallic salt solution | CuCl2 + NaOH ⟶ ............... + ............... ↓ |
| FeCl3 + NaOH ⟶ ............... + ............... ↓ | |
| 26. Ammonium salts | NH4Cl + NaOH ⟶ ............... + ............... + ............... [g] |
| NH4Cl + Ca(OH)2 ⟶ ............... + ............... + ............... [g] |
Answer
| BASES — Ions present in bases | |
|---|---|
| a. Alkali — dissociates yielding hydroxyl ions | |
| 16. Sodium hydroxide | NaOH [aq.] ⇌ Na+ + OH- |
| 17. Ammonium hydroxide | NH4OH [aq.] ⇌ NH4+ + OH- |
| b. Classification | |
| 18. Monoacidic base [Acidity = 1 ] | KOH [aq.] ⇌ K+ + OH- |
| 19. Diacidic base [Acidity = 2] | Cu(OH)2 [aq.] ⇌ Cu2+ + 2OH- |
| c. Preparation of bases | |
| 20. From metals | 4Na + O2 ⟶ 2Na2O |
| 21. From metallic oxides and metals | K2O + H2O ⟶ 2KOH |
| 2K + 2H2O ⟶ 2KOH + H2 | |
| 22. From salts | AlCl3 + 3NaOH ⟶ 3NaCl + Al(OH)3 ↓ |
| FeSO4 + 2NaOH ⟶ Na2SO4 + Fe(OH)2 ↓ | |
| 23. By thermal decomposition | ZnCO3 ⟶ ZnO + CO2 [g] |
| 2Pb(NO3)2 ⟶ 2PbO + 4NO2 [g] + O2 [g] | |
| c. Properties of bases | |
| 24. Neutralizes acids | PbO + 2HNO3 ⟶ Pb(NO3)2 + H2O |
| Fe(OH)2 + 2HCl ⟶ FeCl2 + 2H2O | |
| Reaction with | |
| 25. Metallic salt solution | CuCl2 + 2NaOH ⟶ 2NaCl + Cu(OH)2 ↓ |
| FeCl3 + 3NaOH ⟶ 3NaCl + Fe(OH)3 ↓ | |
| 26. Ammonium salts | NH4Cl + NaOH ⟶ NaCl + H2O + NH3 [g] |
| 2NH4Cl + Ca(OH)2 ⟶ CaCl2 + 2H2O + 2NH3 [g] |
| SALTS — Ions present in salts | |
|---|---|
| 27a. Normal salt [formation] | NaOH [excess] + H2SO4 ⟶ ............... + H2O |
| 27b. Acid salt [formation] | NaOH [insufficient] + H2SO4 ⟶ ............... + H2O |
| Preparation of salts — General methods | |
| Direct combination — Synthesis | |
| 28. Iron and Chloride — salt iron [III] Chloride | Fe + Cl2 ⟶ ............... |
| 29. Zinc and sulphur — salt zinc sulphide | Zn + S ⟶ ............... |
| Displacement — Action of dilute acid on active metals | |
| 30. Iron — salt iron [II] sulphate | Fe + H2SO4 ⟶ ............... + ............... [g] |
| 31. Zinc — salt zinc sulphate | Zn + H2SO4 ⟶ ............... + ............... [g] |
| 32. Magnesium — salt magnesium chloride | Mg + HCl ⟶ ............... + ............... [g] |
| Precipitation — by double decomposition of two salt solutions | |
| 33. Lead nitrate and sodium chloride | Pb(NO3)2 + NaCl ⟶ ............... + ............... ↓ |
| 34. Calcium chloride and sodium carbonate | CaCl2 + Na2CO3 ⟶ ............... + ............... ↓ |
| Neutralization — Action of dilute acid on insoluble base | |
| 35. Oxide — salt copper sulphate | CuO + H2SO4 ⟶ ............... + ............... |
| 36. Hydroxide — salt copper sulphate | Cu(OH)2 + H2SO4 ⟶ ............... + ............... |
| Neutralization — [Titration] Action of dilute acid on an alkali | |
| 37. Hydroxide — salt sodium chloride | NaOH + HCl ⟶ ............... + ............... |
| 38. Hydroxide — salt ammonium chloride | NH4OH + HCl ⟶ ............... + ............... |
| Action of dilute acid on carbonate & bicarbonate | |
| 39. Carbonate — salt lead nitrate | PbCO3 + HNO3 ⟶ ............... + ............... + ............... [g] |
| 40. Carbonate — salt copper chloride | CuCO3 + HCl ⟶ ............... + ............... + ............... [g] |
| 41. Bicarbonate — salt potassium sulphate | KHCO3 + H2SO4 ⟶ ............... + ............... + ............... [g] |
Answer
| SALTS — Ions present in salts | |
|---|---|
| 27a. Normal salt [formation] | 2NaOH [excess] + H2SO4 ⟶ Na2SO4 + 2H2O |
| 27b. Acid salt [formation] | NaOH [insufficient] + H2SO4 ⟶ NaHSO4 + H2O |
| Preparation of salts — General methods | |
| Direct combination — Synthesis | |
| 28. Iron and Chloride — salt iron [III] Chloride | 2Fe + 3Cl2 ⟶ 2FeCl3 |
| 29. Zinc and sulphur — salt zinc sulphide | Zn + S ⟶ ZnS |
| Displacement — Action of dilute acid on active metals | |
| 30. Iron — salt iron [II] sulphate | Fe + H2SO4 ⟶ FeSO4 + H2 [g] |
| 31. Zinc — salt zinc sulphate | Zn + H2SO4 ⟶ ZnSO4 + H2 [g] |
| 32. Magnesium — salt magnesium chloride | Mg + 2HCl ⟶ MgCl2 + H2 [g] |
| Precipitation — by double decomposition of two salt solutions | |
| 33. Lead nitrate and sodium chloride | Pb(NO3)2 + 2NaCl ⟶ 2NaNO3 + PbCl2 ↓ |
| 34. Calcium chloride and sodium carbonate | CaCl2 + Na2CO3 ⟶ 2NaCl + CaCO3 ↓ |
| Neutralization — Action of dilute acid on insoluble base | |
| 35. Oxide — salt copper sulphate | CuO + H2SO4 ⟶ CuSO4 + H2O |
| 36. Hydroxide — salt copper sulphate | Cu(OH)2 + H2SO4 ⟶ CuSO4 + 2H2O |
| Neutralization — [Titration] Action of dilute acid on an alkali | |
| 37. Hydroxide — salt sodium chloride | NaOH + HCl ⟶ NaCl + H2O |
| 38. Hydroxide — salt ammonium chloride | NH4OH + HCl ⟶ NH4Cl + H2O |
| Action of dilute acid on carbonate & bicarbonate | |
| 39. Carbonate — salt lead nitrate | PbCO3 + 2HNO3 ⟶ Pb(NO3)2 + H2O + CO2 [g] |
| 40. Carbonate — salt copper chloride | CuCO3 + 2HCl ⟶ CuCl2 + H2O + CO2 [g] |
| 41. Bicarbonate — salt potassium sulphate | 2KHCO3 + H2SO4 ⟶ K2SO4 + 2H2O + 2CO2 [g] |
Mention the colour changes observed when the following indicators are added to acids:
(i) Alkaline phenolphthalein solution.
(ii) Methyl orange solution
(iii) Neutral litmus solution
Answer
(i) Pink solution becomes colourless.
(ii) Orange solution changes to pink colour.
(iii) Purple solution changes to red colour.
Which of the following hydroxides is not an alkali — [Choose from the choices A, B, C and D]
(A) ammonium hydroxide
(B) calcium hydroxide
(C) copper hydroxide
(D) sodium hydroxide
Answer
Copper hydroxide [Cu(OH)2]
Reason — Copper hydroxide [Cu(OH)2] is an example of insoluble base and it is not an alkali.
Complete the blanks from the list given:
Ammonia, Ammonium, Carbonate, Carbon dioxide, Hydrogen, Hydronium, Hydroxide, Precipitate, Salt, Water.
A solution X turns blue litmus red, so it must contain (i) ............... ions; another solution Y turns red litmus blue and therefore, must contain (ii) ............... ions. When solutions X and Y are mixed together the products will be a (iii) ............... and (iv) ............... . If a piece of magnesium were put into solution X, (v) ............... gas would be evolved.
Answer
A solution X turns blue litmus red, so it must contain (i) hydronium ions; another solution Y turns red litmus blue and therefore, must contain (ii) hydroxide ions. When solutions X and Y are mixed together the products will be a (iii) salt and (iv) water. If a piece of magnesium were put into solution X, (v) hydrogen gas would be evolved.
Match the following:
| Column A | Column B |
|---|---|
| 1.Acid salt | A. Sodium potassium carbonate |
| 2.Normal salt | B. Alum |
| C. Sodium carbonate | |
| D. Sodium zincate | |
| E. Sodium hydrogen carbonate. |
Answer
| Column A | Column B |
|---|---|
| 1.Acid salt | E. Sodium hydrogen carbonate. |
| 2.Normal salt | C. Sodium carbonate |
Write balanced equation for formation of PbCl2 from Pb(NO3)2 soln. and NaCl soln.
Answer
Pb(NO3)2 + 2NaCl ⟶ PbCl2 + 2NaNO3
What is the term defined : i) A base which is soluble in water.
Answer
Alkali is a base which is soluble in water.
The acid which contains four hydrogen atoms —
- Formic acid
- Sulphuric acid
- Nitric acid
- Acetic acid
Answer
Acetic acid contains four hydrogen atoms.
A black coloured solid which on reaction with dilute sulphuric acid forms a blue coloured solution is:
- Carbon
- Manganese [IV] oxide
- Lead [II] oxide
- Copper [II] oxide
Answer
Copper [II] oxide
Reason — Copper [II] oxide is black in colour and the following reaction takes place when it is treated with dilute sulphuric acid —
CuO + H2SO4 ⟶ CuSO4 + H2O
CuSO4 is a blue coloured soln.
Solution A is a strong acid
Solution B is a weak acid
Solution C is a strong alkali
(i) Which solution contains solute molecules in addition to water molecules?
(ii) Which solution will give a gelatinous white precipitate with zinc sulphate solution? The precipitate disappears when an excess of the solution is added.
(iii) Which solution could be glacial acetic acid solution?
(iv) Give example of a soln. of a weak alkali.
Answer
(i) Solution B — weak acid
Reason — Weak Acid is an acid which dissociates only partially in aqueous solution thereby producing a low concentration of hydrogen [H+] ions [or H3O+ ions]. For example — CH3COOH ⇌ CH3COO- + H+ [contains molecules and ions]
(ii) Solution C — strong alkali
Reason — Alkalis react with certain salt solutions to precipitate insoluble hydroxide. Hence,
ZnSO4 + 2NaOH ⟶ Na2SO4 + Zn(OH)2 [gelatinous white precipitate]
(iii) Solution B — weak acid
Reason — Anhydrous acetic acid on cooling forms crystals of glacial acetic acid and acetic acid is a weak acid.
(iv) Ammonium hydroxide (NH4OH)
Write the equation[s] for the reaction[s] to prepare lead sulphate from lead carbonate.
Answer
PbCO3 + 2HNO3 ⟶ Pb(NO3)2 + H2O + CO2
Pb(NO3)2 + Na2SO4 ⟶ PbSO4 + 2NaNO3
Define the following terms — Neutralization
Answer
Neutralization — It is the process due to which [H+] ions of an acid react completely or combine with [OH-] ions of a base to give salt and water only.
Acid + Base ⟶ Salt + Water
HCl + NaOH ⟶ NaCl + H2O
H+Cl- + Na+OH- ⟶ Na+Cl- + H2O
[H+ (aq) + OH- (aq) ⇌ H2O (l)]
A: Nitroso Iron [II] sulphate
B: Iron [III] chloride
C: Chromium sulphate
D: Lead [II] chloride
E: Sodium chloride.
Select from A, B, C, D and E —
(i) A compound soluble in hot water but insoluble in cold water.
(ii) A compound which in the aqueous solution state, is neutral in nature.
Answer
(i) D: Lead [II] chloride
(ii) E: Sodium chloride
Select the correct answer from A, B, C and D –
(i) A weak organic acid is:
- Formic acid
- Sulphuric acid
- Nitric acid
- Hydrochloric acid
(ii) A complex salt is :
- Zinc sulphate
- Sodium hydrogen sulphate
- Iron (II) ammonium sulphate
- Tetrammine copper (II) sulphate
Answer
(i) Formic acid
(ii) Tetrammine copper (II) sulphate
Give an equation for the conversions
(i) ZnSO4 to ZnCO3
(ii) ZnCO3 to Zn(NO3)2
Answer
(i) ZnSO4 + (NH4)2CO3 ⟶ (NH4)2SO4 + ZnCO3
(ii) ZnCO3 + 2HNO3 ⟶ Zn(NO3)2 + H2O + CO2
- NaOH soln.
- Weak acid
- Dil. H2SO4
Select the one which contains solute ions and molecules.
Answer
Weak acid
Reason — An acid which dissociates only partially in aqueous solution thereby producing a low concentration of hydrogen [H+] ions [or H3O+ ions] is a weak acid.
Example — CH3COOH ⇌ CH3COO- + H+ [contains molecules and ions]
Give balanced equation/s for the preparation of the following salts:
- Copper [II] sulphate from CuO.
- Iron [III] chloride from Fe.
- K2SO4 from KOH soln.
- Lead [II] chloride from PbCO3 [give two equations].
Answer
CuO + H2SO4 ⟶ CuSO4 + H2O
2Fe + 3Cl2 ⟶ 2FeCl3
2KOH + H2SO4 [dil.] ⟶ K2SO4 + 2H2O
PbCO3 + 2HNO3 ⟶ Pb(NO3)2 + H2O + CO2
Pb(NO3)2 + 2NaCl ⟶ 2NaNO3 + PbCl2
Write the balanced chemical equation : Lead nitrate solution is added to sodium chloride solution
Answer
Pb(NO3)2 + 2NaCl ⟶ PbCl2 + 2NaNO3
Name the method used from the list:
A : Simple displacement
B : Neutralization
C : Decomposition by acid
D : Double decomposition
E : Direct synthesis
For preparation of the following salts –
(i) Sodium nitrate
(ii) Iron (III) chloride
(iii) Lead chloride
(iv) Zinc sulphate
(v) Sodium hydrogen sulphate.
Answer
(i) Sodium nitrate — B : Neutralization
(ii) Iron (III) chloride — E : Direct synthesis
(iii) Lead chloride — D : Double decomposition
(iv) Zinc sulphate — A : Simple displacement
(v) Sodium hydrogen sulphate — C : Decomposition by acid
Match the following i.e.,
(1) Acid salt
(2) Double salt — with the correct choice from — A and B
A : Ferrous ammonium sulphate
B : Sodium hydrogen sulphate
Answer
- Acid salt — B : Sodium hydrogen sulphate
- Double salt — A : Ferrous ammonium sulphate
Select the word/s given below which are required to correctly complete the blanks — [ammonia, ammonium, carbonate, carbon dioxide, hydrogen, hydronium, hydroxide, precipitate, salt water] :
(i) A solution M turns blue litmus red, so it must contain (i) ............... ions ; another solution O turns red litmus blue and hence, must contain, (ii) ............... ions.
(ii) When solution M and O are mixed together, the products will be (iii) ............... and (iv) ............... .
(iii) If a piece of magnesium was put into a solution M,(v) ............... gas would be evolved.
Answer
(i) A solultion M turns blue litmus red, so it must contain (i) hydronium ions ; another solution O turns red litmus blue and hence, must contain, (ii) hydroxide ions.
(ii) When solution M and O are mixed together, the products will be (iii) salt and (iv) water.
(iii) If a piece of magnesium was put into a solution M, (v) hydrogen gas would be evolved.
Give a suitable chemical term for:
(i) A salt formed by incomplete neutralisation of an acid by a base.
(ii) A definite number of water molecules bound to some salts.
Answer
(i) Acid salt
(ii) Water of crystallization
Choosing the substances from the list given:
dil. Sulphuric acid, Copper, Iron, Sodium, Copper (II) carbonate, Sodium carbonate, Sodium chloride, Zinc nitrate
Write balanced equations for the reactions which would be used in the laboratory to obtain the following salts:
- Sodium sulphate
- Zinc carbonate
- Copper (II) sulphate
- Iron (II) sulphate.
Answer
Sodium sulphate
Na2CO3 + H2SO4 (dil.) ⟶ Na2SO4 + H2O + CO2Zinc carbonate
Zn(NO3)2 + Na2CO3⟶ 2NaNO3 + ZnCO3Copper (II) sulphate
CuCO3 + H2SO4 (dil.) ⟶ CuSO4 + H2O + CO2Iron (II) sulphate
Fe + H2SO4 (dil.) ⟶ FeSO4 + H2
Identify: An acid which is present in vinegar.
Answer
Acetic acid
Fill in the blank from the choices given:
The basicity of acetic acid is ............... [3, 1, 4].
Answer
The basicity of acetic acid is 1.
Draw the structure of the stable positive ion formed when an acid dissolves in water.
Answer
Hydronium ion is the stable positive ion formed when an acid dissolves in water. Its structure is shown below:
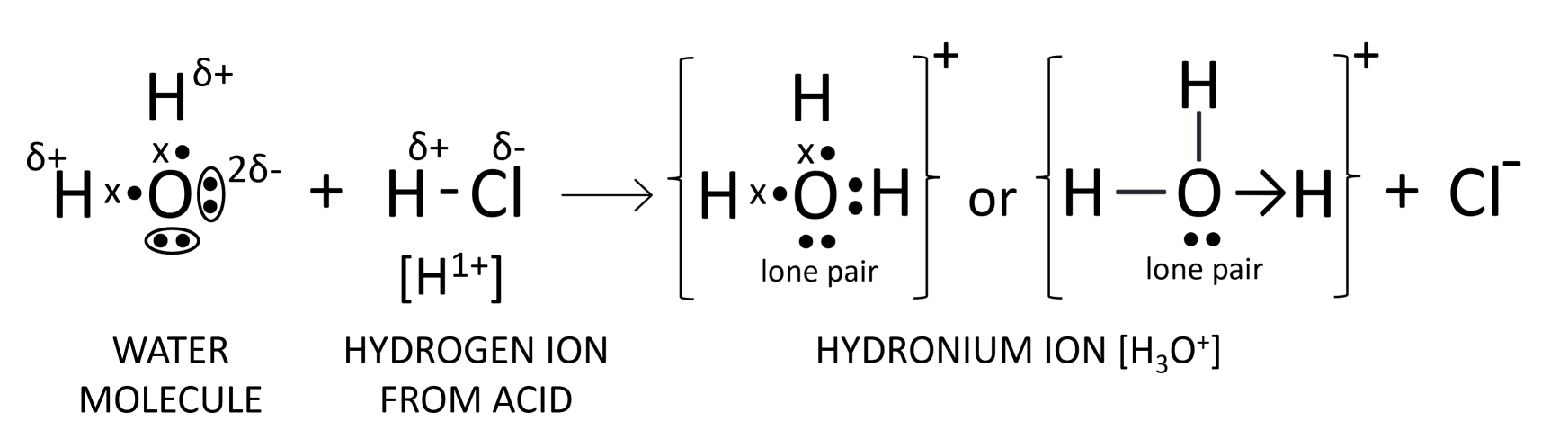
State the inference drawn from the observation:
Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Identify S.
Answer
Salt S is Copper sulphate CuSO4
CuO + H2SO4 (dil.) ⟶ CuSO4 + H2O
Give balanced chemical equations for the preparation of the following salts:
- Lead sulphate — from lead carbonate.
- Sodium sulphate — using dilute sulphuric acid.
- Copper chloride — using copper carbonate.
Answer
Lead sulphate from lead carbonate.
PbCO3 + 2HNO3 ⟶ Pb(NO3)2 + H2O + CO2
Pb(NO3)2 + Na2SO4 ⟶ 2NaNO3 + PbSO4Sodium sulphate using dilute sulphuric acid.
Na2CO3 + H2SO4(dil.) ⟶ Na2SO4 + H2O+ CO2Copper chloride using copper carbonate.
CuCO3 + 2HCl (dil) ⟶ CuCl2 + H2O + CO2
Give a balanced chemical equation for the following conversion.
Fe ⟶ FeCl3
Answer
2Fe + 3Cl2 ⟶ 2FeCl3
From the list of salts — AgCl, MgCl2, NaHSO4, PbCO3
Choose the salt that most appropriately fits the description given below :
An insoluble chloride.
Answer
AgCl
From — SO2, SiO2, Al2O3, MgO, CO, Na2O — Select an oxide which dissolves in water forming an acid.
Answer
SO2
The following reaction takes place.
SO2 + H2O ⟶ H2SO3
Fill in the blank:
Higher the pH value of a solution, the more ............... [acidic/alkaline] it is.
Answer
Higher the pH value of a solution, the more alkaline it is.
Match the following salts given below:
(i) Pb(NO3)2 from PbO
(ii) MgCl2 from Mg
(iii) FeCl3 from Fe
(iv) NaNO3 from NaOH
(v) ZnCO3 from ZnSO4
With their correct method of preparation from: A, B, C, D and E.
(A) Simple displacement
(B) Titration
(C) Neutralization
(D) Precipitation
(E) Combination
Answer
(i) Pb(NO3)2 from PbO — (C) Neutralization
(ii) MgCl2 from Mg — (A) Simple displacement
(iii) FeCl3 from Fe — (E) Combination
(iv) NaNO3 from NaOH — (B) Titration
(v) ZnCO3 from ZnSO4 — (D) Precipitation
Fill in the blanks from the choices given in brackets —
When a metallic oxide is dissolved in water, the solution formed has a high concentration of ............... ions. [H+, H3O+, OH-]
Answer
When a metallic oxide is dissolved in water, the solution formed has a high concentration of OH- ions.
Choose the correct answer from the options —
(i) To increase the pH value of a neutral solution, we should add :
- An acid
- An acid salt
- An alkali
- A salt
(ii) Anhydrous iron [III] chloride is prepared by:
- Direct combination
- Simple displacement
- Decomposition
- Neutralization
Answer
(i) An alkali
Reason — The pH of alkali solutions is more than 7 therefore in order to increase the pH value of a neutral solution, an alkali should be added.
(ii) Direct combination
Reason — Anhydrous iron [III] chloride is prepared by direct combination of iron and chloride as follows —
2Fe + 3Cl2 ⟶ 2FeCl3
Write a balanced chemical equation for the preparation of each of the following salts:
(i) Copper carbonate
(ii) Ammonium sulphate crystals.
Answer
(i) CuSO4 + Na2CO3 ⟶ Na2SO4 + CuCO3
(ii) 2NH4OH + H2SO4 ⟶ (NH4)2SO4 + 2H2O
Give one word or a phrase for the statement :
The property by which certain hydrated salts, when left exposed to the atmosphere, lose their water of crystallization and crumble into powder.
Answer
Efflorescence
State one relevant observation for the following :
Anhydrous calcium chloride is exposed to air for some time.
Answer
It absorbs moisture from the atmosphere, dissolve in the same and change into a solution. This property is known as deliquescence
Fill in the blank with the correct choice given in the bracket —
The salt prepared by the method of direct combination is ............... [iron [II] chloride / iron [III] chloride]
Answer
The salt prepared by the method of direct combination is iron [III] chloride
Three solutions P, Q, and R have pH value of 3.5, 5.2 and 12.2 respectively. State which one of these is a:
(i) weak acid
(ii) strong alkali
Answer
(i) Q
Reason — On a pH scale, acids have pH less than 7 whereas weak acids have pH towards 7. Hence, Q will be a weak acid with pH 5.2 .
(ii) R
Reason — On a pH scale, alkali have pH more than 7 and alkalinity increases as the pH value moves away from 7. Hence, R will be a strong alkali with pH 12.2 .
Write a balanced equation for the preparation of each of the following salts :
(i) Copper [II] sulphate from copper carbonate
(ii) Zinc carbonate from zinc sulphate
Answer
(i) CuCO3 + H2SO4 ⟶ CuSO4 + H2O + CO2
(ii) ZnSO4 + (NH4)2CO3 ⟶ (NH4)2SO4 + ZnCO3
Give the appropriate term defined by the statement given :
The substance that releases hydronium ion as the only positive ion when dissolved in water.
Answer
Acid
Reason — An acid is a compound which when dissolved in water yields hydronium ions [H3O+] as the only positively charged ion.
HCl (aq) ⇌ H+ + Cl-
H+ + H2O ⇌ H3O+ [hydronium ion]
HCl + H2O ⇌ H3O+ + Cl-
The pH values of three solutions A, B, C are given .
Solution A : pH value 12
Solution B : pH value 2
Solution C : pH value 7
Answer the following questions :
(i) Which solution will have no effect on litmus solution.
(ii) Which solution will liberate CO2 when reacted with sodium carbonate.
(iii) Which solution will turn red litmus solution blue.
Answer
(i) Solution C : pH value 7
(ii) Solution B : pH value 2
(iii) Solution A : pH value 12
Choose the method of preparation of the following salts, from the methods given in the list:
List —
A: Neutralization
B: Precipitation
C: Direct combination
D: Substitution
(i) Lead chloride
(ii) Iron [II] Sulphate
(iii) Sodium nitrate
(iv) Iron [III] chloride
Answer
(i) Lead chloride — B: Precipitation
(ii) Iron [II] Sulphate — D: Substitution
(iii) Sodium nitrate — A: Neutralization
(iv) Iron [III] chloride — C: Direct combination
Fill in the blanks from the choices given : A salt prepared by displacement reaction is ............... [Ferric chloride, ferrous chloride, silver chloride]
Answer
Ferrous chloride
Complete the following by selecting the correct options from the choices :
pH of acetic acid is greater than dilute sulphuric acid. So, acetic acid contains ............... concentration of H+ ions. [greater, same, low]
Answer
pH of acetic acid is greater than dilute sulphuric acid. So, acetic acid contains low concentration of H+ ions.
Differentiate between the following pairs based on the information given in the brackets :
Acid and alkali [formation of type of ions]
Answer
| Acid | Alkali |
|---|---|
| An acid is a compound which when dissolved in water yields hydronium ions [H3O+] as the only positively charged ion. | An alkali is a compound which when dissolved in water yields hydroxyl ions [OH-] as the only negatively charged ions. |
Write balanced chemical equations, for the preparation of given salts (i) to (iii) by using the methods A to C respectively.
A: Neutralization
B: Precipitation
C: Titration
(i) Copper sulphate
(ii) Zinc carbonate
(iii) Ammonium sulphate
Answer
Preparation of copper sulphate by neutralization
CuO + H2SO4 ⟶ CuSO4 + H2OPreparation of zinc carbonate by precipitation
Zn(NO3)2 + Na2CO3 ⟶ 2NaNO3 + ZnCO3Preparation of ammonium sulphate by titration
2NH4OH + H2SO4 ⟶ (NH4)2SO4 + 2H2O
Define the following as per ionic theory with examples and ionic equations wherever relevant :
(i) acid (ii) base (iii) alkali (iv) neutralization
Answer
(i) Acid — An acid is a compound which when dissolved in water yields hydronium ions [H3O+] as the only positively charged ion.
HCl (aq) ⇌ H+ + Cl-
H+ + H2O ⇌ H3O+ [hydronium ion]
HCl + H2O ⇌ H3O+ + Cl-
(ii) Base — A base is a compound which reacts with hydronium ions of an acid to give salt and water only.
CuO + 2HCl ⟶ CuCl2 + H2O
Cu(OH)2 + H2SO4 ⟶ CuSO4 + 2H2O
Bases are oxides or hydroxides of a metal [including ammonium hydroxide]
Examples of insoluble bases [i.e., not alkalis] — ZnO, PbO, CuO, Fe(OH)2, Pb(OH)2, Cu(OH)2
(iii) Alkali — An alkali is a compound which when dissolved in water yields hydroxyl ions [OH-] as the only negatively charged ions.
NaOH [aq.] ⇌ Na+ + OH- [Hydroxyl or hydroxide ion]
Alkali is a base, soluble in water. [All alkalis are bases, but all bases are not alkalis.]
Examples of soluble bases [i.e., alkalis] — KOH, NaOH [strong alkalis] , Ca(OH)2, NH4OH (weak alkalis).
(iv) Neutralization — It is the process due to which [H+] ions of an acid react completely or combine with [OH-] ions of a base to give salt and water only.
Acid + Base ⟶ Salt + Water
HCl + NaOH ⟶ NaCl + H2O
H+Cl- + Na+OH- ⟶ Na+Cl- + H2O
[H+ (aq) + OH- (aq) ⇌ H2O (l)]
Differentiate between:
(i) Organic and inorganic acids.
(ii) Hydracids and oxyacids with examples.
Answer
(i) Difference between organic and inorganic acids are as follows :
| Organic acids | Inorganic acids |
|---|---|
| Acids derived from plants, e.g., citric acid, tartaric acid, acetic acid. | Acids derived from minerals e.g. HCl, H2SO4, HNO3 |
(ii) Difference between hydracids and oxyacids are as follows :
| Hydracids | Oxyacids |
|---|---|
| Acids containing hydrogen and a non-metallic element other than oxygen, e.g. HCl, HBr, HI. | Acids containing hydrogen, another element and oxygen, e.g. HNO3, H2SO4. |
State on what basis does the strength of an acid and an alkali depend on.
Answer
Strength of acids depends on the concentration of hydronium ion [H3O+] present in an aqueous solution of an acid.
Strength of alkali depends on the concentration of the hydroxyl ions [OH-] present in an aqueous solution of the alkali.
Differentiate between (i) strong and weak acid (ii) strong and weak alkali with suitable examples and ionic equations.
Answer
(i) Differences between strong and weak acid are as follows :
| Strong Acid | Weak Acid |
|---|---|
| Strong Acid is an acid which dissociates almost completely in aqueous solution there by producing a high concentration of hydrogen [H+] ions [or H3O+ ions] | Weak Acid is an acid which dissociates only partially in aqueous solution thereby producing a low concentration of hydrogen [H+] ions [or H3O+ ions]. |
| HNO3 + H2O ⇌ H3O+ + NO3- [contains almost only ions] | CH3COOH ⇌ CH3COO- + H+ [contains molecules and ions] |
| Examples : Hydrochloric, Sulphuric and Nitric acid. | Examples : Acetic, citric, carbonic, and formic acid. |
(ii) Differences between strong alkali and and weak alkali are as follows :
| Strong alkali | Weak Alkali |
|---|---|
| Strong Alkali is an alkali which dissociates almost completely in aqueous solution thereby producing a high concentration of hydroxyl [OH-] ions. | Weak Alkali is an alkali which dissociates only partially in aqueous solution thereby producing a low concentration of hydroxyl [OH-] ions. |
| NaOH [aq.] ⇌ Na+ + OH- [contains almost only ions] | NH4OH [aq.] ⇌ NH4+ + OH- [contains molecules and ions] |
| Examples : Lithium, Sodium and Potassium hydroxide | Examples : Ammonium hydroxide and Calcium hydroxide. |
Name the ions formed when — HCl; HNO3; H2SO4; CH3COOH; NaOH and NH4OH ionise in aq. soln.
Answer
(i) When HCl is dissolved in water, it is ionised into hydrogen ion [or H3O+ ion] and chloride ion.
HCl ⟶ H+ + Cl-
The H+ cannot exist independently, therefore, it combines with water molecule to form hydronium ion (H3O+)
H+ + H2O ⟶ H3O+
(ii) When HNO3 is dissolved in water, it is ionised into hydronium ion and nitrate ion.
HNO3 ⟶ H+ + NO3-
H+ + H2O ⟶ H3O+
(iii) When H2SO4 is dissolved in water, it is ionised into hydronium ion and sulphate ion.
H2SO4 ⟶ 2H+ + SO42-
H+ + H2O ⟶ H3O+
(iii) When CH3COOH is dissolved in water, it is ionised into hydronium ion and acetate ion.
CH3COOH ⟶ CH3COO- + H+
H+ + H2O ⟶ H3O+
(iii) When NaOH is dissolved in water, it is ionised into sodium ion and hydroxyl ion.
NaOH ⟶ Na+ + OH-
(iv) When NH4OH is dissolved in water, it is ionised into ammonium ion and hydroxyl ion.
NH4OH ⟶ NH4+ + OH-
State giving reasons which is a stronger acid — dil. HCl or conc. H2CO3.
Answer
Dilute HCl is a stronger acid than concentrated H2CO3
Reason — HCl dissociates almost completely in aqueous solution and produces a high concentration of H+ ions and Cl- ions, hence is a strong acid. Whereas, H2CO3 is a weak acid because it dissociates partially yielding H+ ions and bicarbonate HCO3- ion and hence, contains ions as well as molecules. Therefore, dil. HCl is a stronger acid than conc. H2CO3.
State why the basicity of acetic acid is one and acidity of calcium hydroxide is two.
Answer
Basicity of acid — is the number of hydrogen ions [H+] which can be produced per molecule of the acid in aq. soln. Acetic acid [CH3COOH] ionises in aq. soln. and gives one hydrogen ion per molecule of the acid, hence acetic acid is monobasic i.e., it's basicity is one.
Acidity of base — is the number of hydroxyl ions [OH-] which can be produced per molecule of the base in aq. soln. Calcium hydroxide Ca(OH)2 ionises in aq. soln. and gives two hydroxyl ions per molecule of the base, hence calcium hydroxide is a diacidic base i.e., it's acidity is two.
Give three reasons with equations wherever required, why Sulphuric acid is a dibasic acid.
Answer
Sulphuric acid (H2SO4) is a dibasic acid as :
- It ionises in aq. soln. to produce two hydrogen ions per molecule of the acid.
- It dissociate in two steps in aq. soln. as shown below:
H2SO4 + H2O ⇌ H3O+ + HSO4-
HSO4- + H2O ⇌ H3O+ + SO42-
H2SO4 + 2H2O ⇌ 2H3O+ + SO42- - It contains two replaceable hydrogen ions per molecule of the acid so forms two types of salt [acid and normal salt] :
NaOH + H2SO4 ⟶ NaHSO4 (Acid Salt) + H2O
2NaOH + H2SO4 ⟶ Na2SO4 (Normal Salt) + 2H2O
State how acids are defined as per Arrhenius's and Lowry – Bronsted's theory.
Answer
Arrhenius Theory — Acids are substances which dissociate in aqueous solution to give H+ ions.
Strong acids dissociate almost completely, while weak acids dissociate partially.
Lowry – Bronsted's theory — Acids are proton donors and bases are proton acceptors [proton = H+].
HCl [aq.] ⟶ H+ + Cl- [acid - proton donors]
NH3 + H+ ⟶ NH4+ [bases - proton acceptors]
Oxygen atom in water has two 'lone pair of electrons'. Explain the meaning of the term in italics. With the help of an electron dot diagram show the formation of hydronium ion and ammonium ion from a water molecule and an ammonia molecule respectively.
Answer
Oxygen atom in water has two 'lone pair of electrons' implies that two pairs of electrons on oxygen are not shared with any other atom as shown below:
Formation of hydronium ion
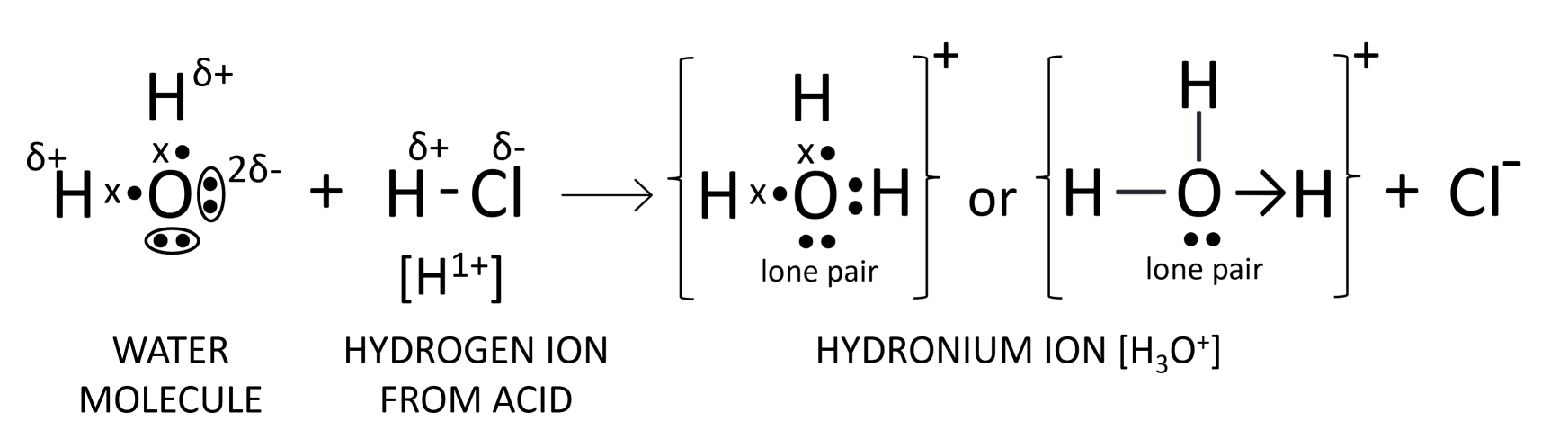
Formation of ammonium ion

State how you would obtain:
- Sulphuric acid from an acidic oxide
- KOH from a basic oxide.
Answer
- Acidic oxides dissolve in water to give an acid.
SO3 + H2O ⟶ H2SO4 - Basic oxides [soluble] dissolve in water to give a base i.e., alkali.
K2O + H2O ⟶ 2KOH
State two chemical properties each with equations of a solution containing
(i) H+ ions
(ii) OH- ions
Answer
(i) Chemical properties of a solution containing H+ (acids) are as follows —
- Neutralization — acids neutralizes base to give salt and water only.
Example : CuO + H2SO4 ⟶ CuSO4 + H2O - Reaction with active metals — Acids react with active metals [e.g., Mg, Al, Zn, Fe] to liberate hydrogen.
Example : Zn + 2HCl ⟶ ZnCl2 + H2
(ii) Chemical properties of a solution containing OH- (bases/alkalis) are as follows —
- Neutralization — Alkalis neutralize acids to form salt and water.
Example :
Ca(OH)2 + 2HCl ⟶ CaCl2 + 2H2O - Reaction with metallic salt — Alkalis react with certain matallic salt solutions to precipitate insoluble hydroxide.
Example :
FeCl3 + 3NaOH ⟶ 3NaCl + Fe(OH)3 ↓ red brown ppt.
Give equations for the decomposition of a metallic (i) chloride (ii) nitrate with conc. H2SO4.
Answer
(i) Decomposition of a metallic chloride —
(ii) Decomposition of a metallic nitrate with conc. H2SO4 —
State in the above reactions a reason for the formation of the respective acids from conc. H2SO4.
Answer
As H2SO4 is a less volatile acid and displaces the more volatile acid on heating with the salt, hence the respective acids are formed.
AB [salt I] + HX [acid I] ⟶ AX [salt II] + HB [acid II]
Convert (i) NaHCO3 (ii) Na2CO3 to unstable carbonic acid by action with dil. H2SO4.
State the reason why ammonia is evolved when an ammonium salt and alkali are heated.
Answer
2NaHCO3 + H2SO4 [dil.] ⟶ Na2SO4 + 2H2O + 2CO2 ⟶ H2CO3 (unstable)
Na2CO3 + H2SO4 [dil.] ⟶ Na2SO4 + H2O + CO2 ⟶ H2CO3 (unstable)
Ammonia is evolved when an ammonium salt and alkali are heated because a less volatile base (e.g.,NaOH) displace the more volatile base, NH4OH and we get the products NH3 and H2O.
NH4Cl + NaOH ⟶ NaCl + H2O + NH3
Define pH value. What would you say about the pH of a solution in which
(i) H+ aq. ions = OH- ions
(ii) evolves CO2 when heated with Na2CO3
(iii) OH-ions > H+ aq. ions.
Answer
pH is defined as the negative logarithm [to the base 10] of the hydrogen ion concentration expressed in moles/litre. Thus, pH = -log10H+. It represents the strength of acids and alkalis, expressed in terms of hydrogen ion concentration [H+ aq.]
(i) When, H+ ions = OH- ions, the solution is neutral with pH = 7.
(ii) When the solution evolves CO2 when heated with Na2CO3, it is acidic in nature with pH less than 7.
(iii) when, OH-ions > H+ aq. ions, the solution is basic in nature with pH more than 7.
State whether litmus is a common acid-base indicator or a universal indicator.
Answer
Litmus is a common acid-base indicator and not a universal indicator. It only indicates whether a solution is acidic or alkaline. It cannot be utilized for determining the strength of the acidic or alkaline solution.
State the colour change in a neutral litmus in presence of (i) acidic (ii) alkaline medium.
Answer
(i) A neutral litmus is purple in colour. In presence of an acidic medium, the colour of neutral litmus changes from purple to red.
(ii) In an alkaline medium, colour of neutral litmus changes from purple to blue.
State the colour change in a universal indicator e.g. pH paper on
(i) slightly acidic soil
(ii) slightly alkaline soil
(iii) dairy milk
(iv) human blood tested for medical diagnosis.
Answer
(i) The pH paper changes to yellow colour on a slightly acidic soil.
(ii) The pH paper changes to blue colour on a slightly alkaline soil.
(iii) The pH paper changes to green colour in a dairy milk.
(iv) The pH paper changes to green colour in human blood tested for medical diagnosis.
Define (i) salt (ii) normal salt (iii) acid salt – with relevant examples and equations.
Answer
(i) Salt — A salt is a compound formed by partial or complete replacement of the replaceable hydrogen ions of an acid by a metallic ion or ammonium ion [basic radical].
NaOH + H2SO4 ⟶ NaHSO4 + H2O [Partial replacement]
2NaOH + H2SO4 ⟶ Na2SO4 + 2H2O [Complete replacement]
(ii) Normal salt — The salt formed by complete replacement of the replaceable hydrogen ion of an acid molecule by a basic radical [metallic or ammonium ion].
For example,
2NaOH + H2SO4 ⟶ Na2SO4 + 2H2O
2NaOH + H2SO3 ⟶ Na2SO3 + 2H2O
[Both H ions in sulphuric and sulphurous acid are replaced by metallic radical — sodium. ]
(3) Acid salt — The salt formed by partial replacement of the replaceable hydrogen ion of an acid molecule by a basic radical [metallic or ammonium ion]. For example,
NaOH + H2SO4 ⟶ NaHSO4 + H2O
NaOH + H2SO3 ⟶ NaHSO3 + H2O
[Only one H ion in sulphuric and sulphurous acid is replaced by metallic radical — sodium. ]
State (i) the formation (ii) the components of – a basic salt.
State which of following salts is an – acid, normal or basic salt.
(i) bleaching powder
(ii) potassium mercuric iodide
(iii) sodium sulphite
(iv) sodium hydrogen sulphite
(v) sodium silver cyanide
(vi) basic lead nitrate
(vii) potassium zincate
(viii) alum
(ix) calcium bicarbonate
(x) basic copper chloride
(xi) trisodium phosphate.
Answer
(i) Formation of a basic salt — A basic salt is formed by partial replacement of hydroxyl radicals of a diacidic or triacidic base with an acid radical.
(ii) Components of a basic salt — A basic salt contains a cation [metallic], a hydroxyl ion [of a base] and an anion [of an acid].
For example — Basic copper nitrate Cu[OH]NO3 , basic copper chloride Cu[OH]Cl
Acid, normal or basic salt —
(i) bleaching powder — Normal salt (Mixed salt)
(ii) potassium mercuric iodide — Normal salt (Complex salt)
(iii) sodium sulphite — Normal salt
(iv) sodium hydrogen sulphite — Acid salt
(v) sodium silver cyanide — Normal salt (Complex salt)
(vi) basic lead nitrate — Basic salt
(vii) potassium zincate — Normal salt
(viii) alum — Normal salt (Double salt)
(ix) calcium bicarbonate — Acid salt
(x) basic copper chloride — Basic salt
(xi) trisodium phosphate — Normal salt
Name three (i) sulphates (ii) chlorides insoluble in water and two (i) oxides (ii) carbonates soluble in water.
Answer
Insoluble in Water
(i) Three sulphates insoluble in water are:
- Lead sulphate (PbSO4)
- Calcium sulphate (CaSO4)
- Barium sulphate (BaSO4).
(ii) Three chloride insoluble in water are:
- Silver chloride (AgCl)
- Lead chloride (PbCl2)
- Mercury chloride (Hg2Cl2).
Soluble in Water
(i) Two oxides soluble in water are:
- Sodium oxide (Na2O)
- Potassium oxide (K2O)
(ii) Two carbonates soluble in water are:
- Sodium carbonate (Na2CO3)
- Ammonium carbonate [(Na4)2CO3]
State the method only, generally used for the preparation of the following salts
(i) Zn(NO3)2
(ii) NH4Cl
(iii) ZnSO4
(iv) ZnS
(v) CaCO3
(vi) FeCl3
(vii) PbCl2
(viii) Pb(NO3)2
Answer
Method of preparation are as follows :
(i) Zn(NO3)2 — By action of dilute acids on carbonate.
(ii) NH4Cl — By neutralisation of an alkali (titration)
(iii) ZnSO4 — By displacement of active metal and acid
(iv) ZnS — Direct combination (Synthesis)
(v) CaCO3 — By precipitation (double decomposition)
(vi) FeCl3 — Direct combination (Synthesis)
(vii) PbCl2 — Precipitation (double decomposition)
(viii) Pb(NO3)2 — Action of dil. acid on carbonates and bicarbonates
Give balanced equations for the preparation of the following salts –
(a) (i) CuSO4
(ii) NaHSO4
(iii) Na2SO4
(iv) FeSO4
(v) BaSO4
(vi) PbSO4 — using dil. H2SO4
(b) (i) NaHSO4
(ii) CuSO4 — using conc. H2SO4
Answer
(a) Using dil. H2SO4 :
- Preparation of CuSO4:
CuO + H2SO4 ⟶ CuSO4 + H2O - Preparation of NaHSO4:
NaOH + H2SO4 ⟶ NaHSO4 + H2O - Preparation of Na2SO4:
Na2CO3 + H2SO4 ⟶ Na2SO4 + H2O + CO2 - Preparation of FeSO4:
Fe + H2SO4 ⟶ FeSO4 + H2 - Preparation of BaSO4:
BaCl2 + H2SO4 ⟶ BaSO4 + 2HCl - Preparation of PbSO4:
Pb(NO3)2 + H2SO4 ⟶ PbSO4 + 2HNO3
(b) Using conc. H2SO4 :
- Preparation of NaHSO4:
- Preparation of CuSO4:
CuO + H2SO4 ⟶ CuSO4 + H2O
Starting from insoluble ZnO how would you obtain insoluble ZnCO3 by precipitation.
Answer
ZnO + 2HCl ⟶ ZnCl2 + H2O
ZnCl2 + Na2CO3 ⟶ ZnCO3 + 2NaCl
Dissolve zinc oxide in dil. HCl. Add to it a saturated solution of Na2CO3. The precipitate formed by the interchange of radicals is filtered. It is dried to obtain the zinc carbonate.
Give balanced equations for the action of a dilute acid on
(i) zinc carbonate,
(ii) potassium bicarbonate for the preparation of the respective salt.
Answer
(i) ZnCO3 + 2HNO3 (dil) ⟶ Zn(NO3)2 + H2O + CO2
(ii) 2KHCO3 + H2SO4 (dil) ⟶ K2SO4 + 2H2O + 2CO2
Give balanced equations for the decomposition of
- calcium bicarbonate by dil. HCl
- calcium carbonate by dil. HNO3
- sodium sulphite by dil. H2SO4
- zinc sulphide by dil. H2SO4.
Answer
- Decomposition of calcium bicarbonate by dil. HCl
Ca(HCO3)2 + 2HCl ⟶ CaCl2 + 2H2O+ 2CO2 - Decomposition of calcium carbonate by dil. HNO3
CaCO3 + 2HNO3 ⟶ Ca(NO3)2 + H2O+ CO2 - Decomposition of sodium sulphite by dil. H2SO4
Na2SO3 + H2SO4 (dil.) ⟶ Na2SO4 + H2O + SO2 - Decomposition of zinc sulphide by dil. H2SO4
ZnS + H2SO4 ⟶ ZnSO4 + H2S
State what will be the effect of each of the following solution on blue litmus —
(i) K2CO3 soln.
(ii) KCl soln.
(iii) NH4NO3 soln.
Answer
(i) As K2CO3 is a salt of a strong base (KOH) and weak acid (H2CO3), it hydrolyses in water to give alkaline solutions. They have pH more than 7, hence it will have no effect on blue litmus.
(ii) KCl is a salt of a strong acid (HCl) and a strong base (KOH). Hence it's aqueous solution will be neutral in nature and will have no effect on blue litmus.
(iii) NH4NO3 is a salt of a strong acid (HNO3) and weak base (NH4OH), it hydrolyses in water to give an acidic solution. They have pH less than 7, hence will turn blue litmus red.
An example of an acid derived from a mineral is ............... [citric acid / nitric acid / acetic acid]
Answer
An example of an acid derived from a mineral is nitric acid.
An example of a base which is not an alkali is ............... [caustic soda / zinc hydroxide / liquor ammonia / caustic potash]
Answer
An example of a base which is not an alkali is zinc hydroxide.
An example of a strong acid is dilute ............... [acetic acid / sulphuric acid / tartaric acid / carbonic acid]
Answer
An example of a strong acid is dilute sulphuric acid.
An example of a weak alkali is ............... [potassium hydroxide / calcium hydroxide / sodium hydroxide] solution.
Answer
An example of a weak alkali is calcium hydroxide solution.
An acid having basicity 1 is ............... [carbonic acid / acetic acid / sulphurous acid]
Answer
An acid having basicity 1 is acetic acid.
An acid obtained by dissolving sulphur trioxide in water is ............... [sulphurous acid / sulphuric acid / oleum]
Answer
An acid obtained by dissolving sulphur trioxide in water is sulphuric acid.
A volatile acid obtained when nitre reacts with non-volatile concentrated sulphuric acid on heating is ............... [hydrochloric acid / sulphuric acid / nitric acid]
Answer
A volatile acid obtained when nitre reacts with non-volatile concentrated sulphuric acid on heating is nitric acid.
A base obtained when lead nitrate undergoes thermal decomposition is ............... [trilead tetroxide / lead (IV) oxide/ lead (II) oxide].
Answer
A base obtained when lead nitrate undergoes thermal decomposition is lead (II) oxide.
An acid obtained when concentrated nitric acid is heated with sulphur is ............... [sulphurous acid / sulphuric acid / nitrous acid]
Answer
An acid obtained when concentrated nitric acid is heated with sulphur is sulphuric acid.
The more volatile acid obtained when the less volatile acid reacts with sodium bicarbonate is ............... [sulphuric acid / carbonic acid / nitric acid]
Answer
The more volatile acid obtained when the less volatile acid reacts with sodium bicarbonate is carbonic acid.
The insoluble base obtained when sodium hydroxide reacts with iron (III) chloride is ............... [iron (II) hydroxide / iron (III) hydroxide / iron (II) oxide]
Answer
The insoluble base obtained when sodium hydroxide reacts with iron (III) chloride is Iron (III) hydroxide.
A solution whose pH is above 7 is ............... [vinegar/milk/liquor ammonia].
Answer
A solution whose pH is above 7 is liquor ammonia.
The salt formed when sulphuric acid reacts with excess caustic soda solution is ............... [sodium bisulphite / sodium sulphate / sodium sulphite / sodium bisulphate].
Answer
The salt formed when sulphuric acid reacts with excess caustic soda solution is sodium sulphate.
An example of an acid salt is ............... [CH3COONa/NaNO3/Na2HPO4/NaKCO3]
Answer
An example of an acid salt is Na2HPO4.
An example of a soluble salt is ............... (AgCl/PbSO4/CaSO4/CaCl2)
Answer
An example of a soluble salt is CaCl2.
An example of an insoluble salt is ............... (Na2CO3/K2CO3/MgCO3/(NH4)2CO3)
Answer
An example of an insoluble salt is MgCO3.
A salt prepared by neutralization in which titration is involved is ............... [MgCl2/CaCl2/NH4Cl/CuCl2]
Answer
A salt prepared by neutralization in which titration is involved is NH4Cl.
An insoluble salt prepared by direct combination or synthesis is ............... [FeCl3/FeSO4/FeS/Fe(NO3)2]
Answer
An insoluble salt prepared by direct combination or synthesis is FeS.
A salt prepared by precipitation i.e. by double decomposition of two salt solutions is ............... [Na2SO4/PbSO4/ZnSO4/CuSO4]
Answer
A salt prepared by precipitation i.e. by double decomposition of two salt solutions is PbSO4.
A salt prepared by simple displacement i.e. action of dilute acid on a metal is ............... [PbCl2/CuCl2/AlCl3/HgCl]
Answer
A salt prepared by simple displacement i.e. action of dilute acid on a metal is AlCl3.
Decomposition of calcium hydrogen carbonate with ............... [dil. HNO3 / dil. HCl / dil. H2SO4] results in formation of calcium chloride.
Answer
Decomposition of calcium hydrogen carbonate with dil. HCl results in formation of calcium chloride.
Action of dilute acid on a metallic sulphide results in evolution of ............... [SO2/H2S/CO2] gas.
Answer
Action of dilute acid on a metallic sulphide results in evolution of H2S gas.
A salt which on hydrolysis produces a neutral solution is ............... (sodium chloride / ammonium chloride / sodium carbonate)
Answer
A salt which on hydrolysis produces a neutral solution is sodium chloride .
Name the following:
- A basic solution which does not contain a metallic element.
- A normal salt of sodium formed from acetic acid.
- A base which reacts with an acid to give a salt which on hydrolysis gives a slightly acidic solution.
- An ion which combines with a polar covalent molecule to form an ammonium ion.
- A soluble salt formed by direct combination between a light metal & a greenish yellow gas.
Answer
- Ammonium Hydroxide (NH4OH) is a basic solution which does not contain a metallic element.
- Sodium acetate (CH3COONa) is a normal salt of sodium formed from acetic acid.
- Ammonium hydroxide (NH4OH) is a base which reacts with an acid to give a salt which on hydrolysis gives a slightly acidic solution.
- Hydrogen ion (H+) is an ion which combines with a polar covalent molecule to form an ammonium ion.
- Aluminium chloride (AlCl3) is a soluble salt formed by direct combination between a light metal & a greenish yellow gas where, light metal is Al and greenish yellow gas is Cl.
Identify which of the following terms matches with the appropriate descriptions 1 to 5.
A: Hydracid
B: Monobasic acid
C: Less volatile acid
D: Weak acid
E: Tribasic acid
F: Dibasic acid
G: More volatile acid
- An acid having basicity 1 and having only one replaceable hydrogen ion per molecule of the acid.
- An acid which dissociates to give a low concentration of H+ ions.
- An acid containing hydrogen and a non-metallic element other than oxygen.
- The type of acid which generally displaces another acid when the acid is heated with a salt.
- The type of acid which reacts with a base to give an acid salt and a normal salt.
Answer
- B: Monobasic acid
- D: Weak acid
- A: Hydracid
- C: Less volatile acid
- F: Dibasic acid
State which of the following methods is generally used for preparing the salts 1 to 5 given below:
A: Neutralisation — insoluble base and dil. acid
B: Neutralisation — alkali and dil. acid
C: Simple displacement — active metal and dil. acid
D: Direct combination
E: Precipitation [double decomposition]
- PbCO3
- Zn(NO3)2
- NaCl
- Cu(NO3)2
- FeS
Answer
- E: Precipitation (Double decomposition)
- C: Simple displacement — active metal and dil. acid
- B: Neutralisation — alkali and dil. acid
- A: Neutralisation — insoluble base and dil. acid
- D: Direct combination
Give balanced equations for the preparation of the following salts:
- Calcium oxide ⟶ Calcium chloride ⟶ Calcium carbonate
- Zinc sulphide ⟵ Zn ⟶ Zinc sulphate
- Iron [II] chloride ⟵ Fe ⟶ Iron [III] chloride
- Lead [II] oxide ⟶ Lead nitrate ⟶ Lead sulphate
- Copper [II] oxide ⟶ Copper [II] sulphate ⟵ Copper [II] hydroxide
Answer
- Calcium oxide ⟶ Calcium chloride ⟶ Calcium carbonate
- CaO + 2HCl ⟶ CaCl2 + H2O
- CaCl2 + Na2CO3 ⟶ 2NaCl + CaCO3
- Zinc sulphide ⟵ Zn ⟶ Zinc sulphate
- Zn + S ⟶ ZnS
- Zn + H2SO4 ⟶ ZnSO4 + H2
- Iron [II] chloride ⟵ Fe ⟶ Iron [III] chloride
- Fe + 2HCl ⟶ FeCl2 + H2
- 2Fe + 3Cl2 ⟶ 2FeCl3
- Lead [II] oxide ⟶ Lead nitrate ⟶ Lead sulphate
- PbO + 2HNO3 ⟶ Pb(NO3)2 + H2O
- Pb(NO3)2 + Na2SO4 ⟶ 2NaNO3 + PbSO4
- Copper [II] oxide ⟶ Copper [II] sulphate ⟵ Copper [II] hydroxide
- CuO + H2SO4 ⟶ CuSO4 + H2O
- Cu(OH)2 + H2SO4 ⟶ CuSO4 + 2H2O
The diagram represents the preparation of sodium sulphate salt from dil. H2SO4 acid and sodium hydroxide.
![The diagram represents the preparation of sodium sulphate salt from dil. H2SO4 acid and sodium hydroxide. Name the apparatus A. Name the substance X placed in A and the substance Y placed in B. State the reason for conducting the titration using the apparatus A and B. State which solution is transferred to the evaporating dish and evaporated to point of crystallization for obtaining the salt. State why titration is not conducted for the preparation of copper [II] sulphate crystals by neutralization. Acids, Bases and Salts, Simplified Chemistry Dalal Solutions ICSE Class 10](https://cdn1.knowledgeboat.com/img/scd10/sodium-sulphate-preparation-simplified-chemistry-icse-class-10-252x178.png)
- Name the apparatus 'A'.
- Name the substance 'X' placed in 'A' and the substance 'Y' placed in B.
- State the reason for conducting the titration using the apparatus 'A' and 'B'.
- State which solution is transferred to the evaporating dish and evaporated to point of crystallization for obtaining the salt.
- State why titration is not conducted for the preparation of copper [II] sulphate crystals by neutralization.
Answer
- Burette
- Dil. sulphuric acid (H2SO4) is substance 'X' placed in 'A' and sodium hydroxide (NaOH) is the substance 'Y' placed in B.
- Titration is conducted to determine the completion of the neutralization reaction, i.e. to determine the amount of sulphuric acid required to neutralize a known amount of sodium hydroxide.
- Aqueous sodium sulphate (Na2SO4.10H2O) is transferred to the evaporating dish and evaporated to point of crystallisation for obtaining the salt.
- As copper (II) oxide is not soluble in water hence titration is not conducted for the preparation of copper (II) sulphate crystals by neutralization.
Give reasons for the following:
Concentrated sulphuric acid is a weaker acid compared to dilute sulphuric acid.
Answer
Strength of an acid is determined by the amount of hydronium ions it produces in it's aqueous solution. As dilute sulphuric acid dissociates almost completely thereby producing a high concentration of hydronium ions hence is a stronger acidic than concentrated sulphuric acid.
Give reasons for the following:
An aqueous solution of the salt ammonium chloride is acidic in nature while an aqueous solution of sodium chloride is neutral.
Answer
As ammonium chloride is a salt of a weak alkali and strong acid, hence it's aqueous solution is acidic in nature. On the other hand, sodium chloride is a product of strong alkali and strong acid hence, it's aqueous solution is neutral in nature.
| Neutralization reaction | Base + Acid | Salt | + water | Nature |
|---|---|---|---|---|
| Weak alkali + strong acid | NH4OH + HCl | ⟶ NH4Cl | + H2O | acidic |
| Strong alkali + strong acid | NaOH + HCl | ⟶ NaCl | + H2O | neutral |
Give reasons for the following:
In the preparation of an insoluble salt from another insoluble salt by precipitation [double decomposition], dilute nitric acid and not dilute sulphuric acid is generally used.
Answer
In the preparation of an insoluble salt from another insoluble salt by precipitation, if dil. sulphuric acid is directly used then it forms an insoluble precipitate and slows the reaction.
For example — Direct addition of dil. sulphuric acid to PbCO3 is an impractical method of preparing lead sulphate since PbSO4 is insoluble and forms a coating on PbCO3, thereby the reaction slowly comes to a stop.
Give reasons for the following:
Acetic acid does not form an acid salt but forms a normal salt.
Answer
Acetic acid (CH3COOH) is a monobasic acid and contains only one replaceable hydrogen atom, hence it can only form normal salt and not acid salts.
Give reasons for the following:
Sulphurous acid forms two types of salts on reaction with an alkali.
Answer
Sulphurous acid (H2SO3) is a dibasic acid, i.e., it contains two replaceable hydrogen ions per molecule of the acid. Hence, it can form normal salt as well as acid salt on reaction with an alkali.