Questions
Question 1(2008)
The formation of 1, 2-dibromoethane from ethene and bromine is an example of :
- Substitution
- Dehydration
- Dehydrohalogenation
- Addition
Answer
Addition
Formation of 1, 2-dibromoethane from ethene and bromine

Question 2(2008)
Name the organic compound prepared by each of the following reactions :
(i) C2H5COONa + NaOH ⟶
(ii) CH3I + 2[H]⟶
(iii) C2H5Br + KOH (alcoholic soln.) ⟶
(iv) CaC2 + 2H2O ⟶
Answer
(i) Ethane
(ii) Methane
(iii) Ethene [ethylene]
(iv) Ethyne [Acetylene]
Question 3(2008)
Write the equation for the following :
(i) Calcium carbide and water
(ii) Ethene and water (steam)
(ii) Bromoethane and an aqueous solution of sodium hydroxide
Answer
(i) Calcium carbide and water: forms ethyne [acetylene]
(ii) Ethene and water (steam): form Ethanol [ethyl alcohol]
(iii) Bromoethane and an aqueous solution of sodium hydroxide: form Ethanol [ethyl alcohol]
Question 4(2008)
Distinguish between ethane [saturated] and ethene [unsaturated] by drawing their structural formulae.
Answer
Structural formula of Ethane [saturated]:

Structural formula of Ethene [unsaturated]:

Question 5(2008)
Which type of reaction i.e. addition or substitution is shown by ethane and ethene ?
Answer
Ethane ⟶ Substitution reaction
The non-availability of electrons in the single covalent bond makes them less reactive and therefore undergo characteristic substitution reaction only.
Ethene ⟶ Addition reaction
The availability of electrons in the double or triple bond makes them more reactive and therefore undergo characteristic addition reactions only.
Question 6(2008)
Write the equation for the complete combustion of ethane.
Answer
2C2H6 + 7O2 [excess] ⟶ 4CO2 + 6H2O + Δ
Question 7(2008)
Name the alcohol, aldehyde and acid formed when ethane is oxidized.
Answer
Alcohol ⟶ Ethyl alcohol [C2H5OH]
Aldehyde ⟶ Acetaldehyde [CH3CHO]
Acid ⟶ Acetic acid [CH3COOH]
Question 8(2008)
Why is pure acetic acid known as glacial acetic acid ?
Answer
Anhydrous acetic acid on cooling below 16.5°C, crystallizes out in the pure form, forming a crystalline mass resembling ice [m.p. around 17 °C] . Hence, pure acetic acid is called glacial acetic acid.
Question 9(2008)
What type of compound is formed by the reaction between acetic acid and an alcohol ?
Answer
Ester is formed by the reaction between acetic acid and an alcohol.
Question 10(2008)
By what type of reaction could a compound containing C, H and Cl be obtained from ethyne ?
Answer
Addition reaction
Reaction of ethyne with chlorine:

Question 11(2008)
State the term for the reaction in which the hydrogen of an alkane is replaced by chlorine.
Answer
Substitution reaction
Question 1(2009)
Which of the following statements is wrong about alkanes ?
(A) They are all saturated hydrocarbons.
(B) They can undergo addition as well as substitution reactions.
(C) They are almost non polar in nature.
(D) On complete combustion give out carbon dioxide and water.
Answer
They can undergo addition as well as substitution reactions.
Reason — The non availability of electrons in the single covalent bond makes them less reactive and therefore they undergo characteristic substitution reactions only.
Question 2(2009)
Write balanced equation for : Acetic acid is warmed with ethanol in the presence of conc. H2SO4.
Answer
Question 3(2009)
Find the odd one out in each case and explain your choice.
(i) C3H8 , C5H10, C2H6, CH4
(ii) Formic acid, Nitric acid, Acetic acid, Propanoic acid.
Answer
(i) C5H10 [Rest all are alkanes and this is an alkene]
(ii) Nitric acid [Rest all are organic acids and this is inorganic acid]
Question 4(2009)
Identity 'S' : Reddish brown liquid 'S' is dissolved in water. When ethyne is passed through it, turns colourless.
Answer
Bromine solution

Question 5(2009)
Fill in the blanks with the correct words from the brackets:
Generally ionic compounds exist in (i) ............... [solid/liquid/gas] state. Melting and boiling points of covalent compounds are generally (ii) ............... [low/high]. The general formula for alkane is (iii) ............... (CnH2n/CnH2n-2/CnH2n+2). For alkynes the general formula is (iv) ............... (CnH2n/ CnH2n-2/CnH2n+2)
Answer
Generally ionic compounds exist in solid state. Melting and boiling points of covalent compounds are generally low. The general formula for alkane is CnH2n+2. For alkynes the general formula is CnH2n-2
Question 6(2009)
Give chemical equation for :
(i) The laboratory preparation of methane from sodium acetate.
(ii) The reaction of one mole of ethene with one mole of chlorine gas.
(iii) The preparation of ethyne from 1, 2 – dibromoethane.
Answer
(i) methane from sodium acetate :
(ii) one mole of ethene with one mole of chlorine gas:

(iii) ethyne from 1, 2 – dibromoethane.

Question 7(2009)
How are the following conversions carried out:
(i) Ethyl chloride to Ethyl alcohol.
(ii) Ethyl chloride to Ethene.
(iii) Ethene to Ethyl alcohol.
(iv) Ethyl alcohol to Ethene.
Answer
(i) By boiling ethyl chloride with aqueous NaOH.
(ii) By boiling ethyl chloride with alcoholic KOH.
(iii) Ethene is absorbed in conc. sulphuric acid at 80 °C under 30 atmos. to give ethyl hydrogen sulphate, which on hydrolysis with steam gives ethanol.
(iv) By heating ethyl alcohol with concentrated H2SO4 at 170°C.
Question 8(2009)
Define isomerism. Give the IUPAC name of the isomer C4H10 which has a branched chain.
Answer
(1) Isomerism is the phenomenon due to which two or more compounds have the same molecular formula but differ in molecular arrangement or in structural formula.
Example : Isomers of pentane are (i) n pentane, (ii) isopentane and (iii) neo-pentane
IUPAC name of C4H10 is Butane.
Question 1(2010)
(i) Select the correct answer - The organic compound, which gives a red precipitate with ammoniacal cuprous chloride and undergoes an addition reaction –
A: Ethane
B: Ethene
C: Ethyne
D: Ethanol
(ii) Select the correct answer - The organic compound which when mixed with ethyl alcohol, [ethanol], makes it spurious.
A: Methanol
B: Methanoic acid
C: Methanal
D: Ethanoic acid
Answer
(i) Ethyne
(ii) Methanol
Question 2(2010)
Draw the structural formula of —
(i) Ethanoic acid (ii) But-2-yne
Answer
(i) Structural formula of Ethanoic acid is shown below:

(ii) Structural formula of But-2-yne is shown below:

Question 3(2010)
Compound 'X' is bubbled through bromine dissolved in CCl4 and the product formed is CH2Br – CH2Br.
i) Draw the structural formula of 'X' and state what type of reaction 'X' has undergone.
(ii) State your observation for the above reaction.
(iii) Name the compound formed when steam reacts with 'X' in the presence of an acid, eg. phosphoric acid.
(iv) What is the procedure for converting the product formed in (iii) above, back to 'X' ?
Answer
(i) Compound 'X' is Ethene (H2C=CH2). Its structural formula is shown below:

Ethene [CH2=CH2] has undergone addition reaction.
(ii) Brown colour of bromine is discharged.
(iii) Ethanol
(iv) Ethanol can be converted into ethene, by dehydrating it with concentrated H2SO4 at 170°C.
Question 1(2011)
Name a gaseous hydrocarbon commonly used for welding purposes.
Answer
Ethyne [Acetylene]
Question 2(2011)
Give reasons for the following –
(i) almost 90% of all known compounds are organic in nature.
(ii) it is dangerous to burn methane in an insufficient supply of air.
Answer
(i) Due to unique nature of carbon atoms, which includes tetravalency, catenation and leading to formation of isomers, millions of organic compounds are known. Hence, almost 90% of all known compounds are organic in nature.
(ii) When methane burns in an insufficient supply of air, carbon monoxide is produced which is extremely poisonous for human beings as it cuts off the oxygen supply and forms carboxy haemoglobin in the blood, leading to death.
Question 3(2011)
Choose the correct answer –
(i) The functional group present in acetic acid is -
A: Ketonic C = O
B: Hydroxyl - OH
C: Aldehydic – CHO
D: Carboxyl – COOH
(ii) Unsaturated hydrocarbons undergo :
A: substitution reaction
B: oxidation reaction
C: addition reaction
D: none of the above
(iii) The number of C – H bonds in ethane molecule are:
A: Four
B: Six
C: Eight
D: Ten
Answer
(i) Carboxyl – COOH
CH3-COOH
(ii) addition reaction
The availability of electrons in the double or triple bond makes them more reactive and therefore undergo characteristic addition reactions only.
(iii) Six
Below is the structural formula of Ethane:

Question 4(2011)
Select the correct answer from the choices given :
(i) The catalyst used for conversion of ethene to ethane is commonly .............. [nickel/iron/cobalt]
(ii) Acetaldehyde when oxidized with acidified potassium dichromate, forms .............. [ester/ethanol/acetic acid]
(iii) Ethanoic acid reacts with ethanol in presence of conc. H2SO4, so as to form a compound and water. The chemical reaction which takes place is called .............. [dehydration/hydrogenation/esterification]
Answer
(i) The catalyst used for conversion of ethene to ethane is commonly nickel.
(ii) Acetaldehyde when oxidized with acidified potassium dichromate, forms acetic acid.
(iii) Ethanoic acid reacts with ethanol in presence of conc. H2SO4, so as to form a compound and water. The chemical reaction which takes place is called esterification
Question 5(2011)
Write balanced chemical equations for the following :
(i) Reaction between 1, 2 – dibromoethane and alcoholic potassium hydroxide.
(ii) Monochloro ethane is hydrolysed with aqueous KOH.
(iii) A mixture of sodalime and sodium acetate is heated.
(iv) Ethanol under high pressure and low temperature is treated with acidified potassium dichromate.
(v) Water is added to calcium carbide.
(vi) Ethanol reacts with sodium at room temperature.
Answer
(i) Reaction between 1, 2 – dibromoethane and alcoholic potassium hydroxide:

(ii) Monochloro ethane is hydrolysed with aqueous KOH:
(iii) A mixture of sodalime and sodium acetate is heated:
(iv) Ethanol under high pressure and low temperature is treated with acidified potassium dichromate:
(v) Water is added to calcium carbide:
(vi) Ethanol reacts with sodium at room temperature:
Question 1(2012)
State the observation : Bromine vapours are passed into a soln. of ethyne in carbon tetrachloride.
Answer
Reddish brown bromine dissolved in water turns colourless when ethyne is passed through it.

Question 2(2012)
From – Ethyne, ethanol, acetic acid, ethene, methane. Choose the one which relates to (i) to (iv).
(i) An unsaturated hydrocarbon used for welding purposes.
(ii) An organic compound whose functional group is carboxyl.
(iii) A hydrocarbon which on catalytic hydrogenation gives a saturated hydrocarbon.
(iv) An organic compound used as a thermometric liquid.
Answer
(i) Ethyne
(ii) Acetic acid
(iii) Ethene
(iv) Ethanol
Question 3(2012)
(i) Why is pure acetic acid known as glacial acetic acid?
(ii) Give a chemical equation for the reaction between ethyl alcohol and acetic acid.
Answer
(i) Anhydrous acetic acid on cooling below 16.5°C, crystallizes out in the pure form, forming a crystalline mass resembling ice [m.p. around 17°C] . Hence, pure acetic acid is called glacial acetic acid.
(ii) Reaction between ethyl alcohol and acetic acid:
Question 4(2012)
Rewrite with the missing word/s:
Ethyl alcohol is dehydrated by sulphuric acid at a temperature of about 170°C.
Answer
Ethyl alcohol is dehydrated by concentrated sulphuric acid at a temperature of about 170°C.
Question 5(2012)
Give the structural formula for the following :
(i) Methanoic acid
(ii) Ethanal
(iii) Ethyne
(iv) Acetone
(v) 2-methyl propane.
Answer
(i) Methanoic acid:

(ii) Ethanal:

(iii) Ethyne:

(iv) Acetone:

(v) 2-methyl propane:

Question 1(2013)
Identify the gas evolved when : sodium propionate is heated with soda lime.
Answer
Ethane gas
Question 2(2013)
Give suitable chemical term for:
A reaction in which hydrogen of an alkane is replaced by a halogen.
Answer
Subsitution reaction
Question 3(2013)
Give a chemical test to distinguish between : Ethene gas and ethane gas.
Answer
When bromine is passed through solutions of each of the gases in an inert solvent [CCl4] at room temperature, incase of ethene gas, brown colour of bromine is discharged whereas in case of ethane gas brown colour remains brown.
Question 4(2013)
Identify the statement that is incorrect about alkanes :
A: They are hydrocarbons.
B: There is a single covalent bond between carbon and hydrogen.
C: They can undergo both substitution as well as addition reactions.
D: On complete combustion they produce carbon dioxide and water.
Answer
They can undergo both substitution as well as addition reactions.
Reason — The non availability of electrons in the single covalent bond makes them less reactive and therefore alkanes undergo characteristic substitution reactions only.
Question 5(2013)
Give balanced equations for the laboratory preparations of:
(i) A saturated hydrocarbon from iodomethane.
(ii) An unsaturated hydrocarbon from an alcohol.
(iii) An unsaturated hydrocarbon from calcium carbide.
(iv) An alcohol from ethyl bromide.
Answer
(i) Methane is produced
(ii) Ethene is formed
(iii) Ethyne is formed
(iv) ethyl alcohol is formed.
Question 6(2013)
Give the structural formulae for :
(i) An isomer of n-butane.
(ii) 2-propanol.
(iii) Diethyl ether.
Answer
(i) Iso-butane is an isomer of n-butane. Its structure is shown below:

(ii) 2-propanol:

(iii) Diethyl ether:

Question 7(2013)
Give reasons for:
(i) Methane does not undergo addition reactions, but ethene does.
(ii) Ethyne is more reactive than ethane.
(iii) Hydrocarbons are excellent fuels.
Answer
(i) Methane is a saturated hydrocarbon whereas Ethene is an unsaturated hydrocarbon. In saturated hydrocarbons, all the four valencies of each carbon atom are satisfied by the hydrogen atoms, forming single covalent bond. Thus, due to non-availability of electrons methane is less reactive and hence does not undergo addition reactions.
On the other hand, ethene has two carbon atoms forming a double covalent bond as their valencies are not fully satisfied by hydrogen atoms. The availability of electrons in the double bond makes ethene more reactive and it undergoes addition reactions.
(ii) Ethyne is an unsaturated hydrocarbon having two carbon atoms forming a triple covalent bond as their valencies are not fully satisfied by hydrogen atoms whereas ethane is a saturated hydrocarbon as all the four valencies of its two carbon atoms are satisfied by the hydrogen atoms. The availability of electrons in the triple bond in case of ethyne makes it more reactive than ethane which has does not have electrons available in the single covalent bond.
(iii) Hydrocarbons have high calorific value. They are easily combustible and the reaction is exothermic releasing heat energy. Hence, they are excellent fuels.
Question 1(2014)
The I.U.P.A.C. name of acetylene is -
A: propane
B: propyne
C: ethene
D: ethyne.
Answer
Ethyne
Question 2(2014)
Ethanol reacts with sodium to give ............... [sodium ethanoate, sodium ethoxide, sodium propanoate]
Answer
Ethanol reacts with sodium to give sodium ethoxide
Question 3(2014)
Give one word or phrase for hydrocarbons containing a functional group
Answer
Ketones
Question 4(2014)
Write balanced equation for preparation of:
(i) ethane from sodium propionate.
(ii) ethanol from monochloroethane and aq. sodium hydroxide.
Answer
(i) Preparation of ethane from sodium propionate.
(ii) Preparation of ethanol from monochloroethane and aq. sodium hydroxide.
Question 5(2014)
Distinguish between Ethane and ethene (using alkaline potassium permanganate solution)
Answer
When ethene is passed through alkaline potassium permanganate solution (cold dil. KMnO4), it decolourizes the purple coloured solutions whereas on passing Ethane, alkaline potassium permanganate solution remains purple.
Question 6(2014)
State the conditions required for :
(i) Catalytic hydrogenation of ethyne.
(ii) Preparation of ethyne from ethylene dibromide.
Answer
(i) Vapours of ethyne mixed with hydrogen are passed over a catalyst eg., nickle [Pd. or Pt.] at high temperatures of around 300°C.
(ii) Ethylene dibromide is boiled with conc. alcoholic KOH soln. in a round bottom flask. Ethyne is collected by downward displacement of water.
Question 7(2014)
Write structural formula of:
(i) ethanol
(ii) 1-propanal.
(iii) ethanoic acid.
(iv) 1, 2, dichloroethane.
Answer
(i) Structural formula of ethanol is shown below:

(ii) Structural formula of 1-propanal is shown below:

(iii) Structural formula of ethanoic acid is shown below:

(iv) Structural formula of 1, 2, dichloroethane is shown below:

Question 8(2014)
Match A and B with (i) and (ii) :
| Column I | Column II |
|---|---|
| A: alkynes | (1) CnH2n+2 |
| B: alkane | (2) CnH2n-2 |
Answer
| Column I | Column II |
|---|---|
| A: alkynes | (2) CnH2n-2 |
| B: alkane | (1) CnH2n+2 |
Question 1(2015)
Select from the list — Ammonia, ethane, hydrogen chloride, hydrogen sulphide, ethyne
(i) The gas is used for welding purposes.
(ii) This gas is also a saturated hydrocarbon.
Answer
(i) Ethyne
(ii) Ethane
Question 2(2015)
State which of the following statements does not describe the property of alkenes :
A: They are unsaturated hydrocarbons
B: They decolourise bromine water
C: They can undergo addition as well as substitution reactions
D: They undergo combustion with oxygen forming carbon dioxide and water.
Answer
They can undergo addition as well as substitution reactions
Reason — The availability of electrons in the double or triple bond makes them more reactive and therefore they undergo characteristic addition reactions only.
Question 3(2015)
State the observation : The gaseous product from dehydration of ethanol is passed through bromine water.
Answer
When the gaseous product from dehydration of ethanol is passed through bromine water, the brown colour of bromine water is discharged.
Question 4(2015)
Give balanced chemical equations for the following conversions:
(i) Ethanoic acid to ethyl ethanoate.
(ii) Calcium carbide to ethyne.
(iii) Sodium ethanoate to methane.
Answer
(i) Ethanoic acid to ethyl ethanoate. :
(ii) Ethyne is formed
(iii) Sodium ethanoate to methane
Question 5(2015)
Using their structural formulae identify the functional group by circling them:
(i) Dimethyl ether.
(ii) Propanone
Answer
(i) Dimethyl ether contains the functional group Alkoxy (ether). It is circled in its structural formula below:

(ii) Propanone contains the functional group Keto. It is circled in its structural formula below:

Question 6(2015)
Name the following :
(i) The process by which ethane is obtained from ethene.
(ii) A hydrocarbon which contributes towards the greenhouse effect.
(iii) The distinctive reaction that takes place when ethanol is treated with acetic acid.
(iv) The property of elements by virtue of which atoms of the element can link to each other in the form of a long chain or ring structure.
(v) The reaction when an alkyl halide is treated with alcoholic potassium hydroxide.
Answer
(i) Catalytic hydrogenation (addition)
(ii) Methane
(iii) Esterification
(iv) Catenation
(v) Dehydrohalogenation
Question 1(2016)
Fill in the blanks : Conversion of ethene to ethane is an example of ............... [hydration/hydrogenation].
Answer
Conversion of ethene to ethane is an example of hydrogenation.
Question 2(2016)
Write balanced chemical equation for : Preparation of ethanol from ethyl chloride.
Answer
Question 3(2016)
Identify the term/substance in each of the following :
(i) The catalyst used in the conversion of ethyne to ethane.
(ii) The type of reactions alkenes undergo.
Answer
(i) Nickel or platinum or palladium.
(ii) Addition reaction
Question 4(2016)
Write the IUPAC names of:

Answer
(a) Propene
(b) 2-butyne
(c) ethanal
Question 5(2016)
Write a balanced equation for :
(i) Burning of ethane in plentiful supply of air.
(ii) Action of water on calcium carbide.
(iii) Heating of ethanol at 170°C in the presence of conc. sulphuric acid.
Answer
(i) Carbon dioxide and water are produced:
(ii) Calcium hydroxide and ethyne are produced
(iii) Ethene and water are produced
Question 6(2016)
Give the structural formulae of:
(i) 2-methyl propane
(ii) Ethanoic acid
(iii) Butan-2-ol
Answer
(i) 2-methyl propane :

(ii) Ethanoic acid :

(iii) Butan – 2 – ol :

Question 7(2016)
Compound A is bubbled through bromine dissolved in carbon tetrachioride as follows :

(i) Draw the structure of A.
(ii) State your observation during this reaction.
Answer
(i) Compound A is Ethene. Its structure is shown below:

(ii) Brown colour of bromine is discharged.
Question 1(2017)
Fill in the blanks from the choices given in brackets – The compound formed when ethene reacts with hydrogen is ............... [CH4, C2H6, C3H8]
Answer
The compound formed when ethene reacts with hydrogen is C2H6
Question 2(2017)
Choose the correct answer from the options given – If the molecular formula of an organic compound is C10H18 it is –
(i) Alkene
(ii) Alkane
(iii) Alkyne
(iv) Not a hydrocarbon
Answer
Alkyne
Reason — As the general formula for alkyne is CnH2n-2. Hence, when C is 10 then H is 18.
Question 3(2017)
Identify the substance underlined – An organic compound containing – COOH functional group.
Answer
Carboxylic acids
Question 4(2017)
Write the balanced chemical equation for – Preparation of methane from iodomethane
Answer
Question 5(2017)
Identify the term or substance based on the descriptions given below:
(i) Ice like crystals formed on cooling an organic acid sufficiently.
(ii) Hydrocarbon containing a triple bond used for welding purposes.
(iii) The property by virtue of which the compound has the same molecular formula but different structural formulae.
(iv) The compound formed where two alkyl groups are linked by group.
Answer
(i) Glacial acetic acid
(ii) Ethyne or acetylene
(iii) Isomerism
(iv) Ketone or Alkanone
Question 6(2017)
Give a balanced chemical equation for each of the following –
(i) Preparation of ethane from sodium propionate.
(ii) Action of alcoholic KOH on bromoethane.
Answer
(i) ethane from sodium propionate : ethane is formed
(ii) ethyl alcohol is formed.
Question 7(2017)
State one relevant observation for – Addition of ethyl alcohol to acetic acid in the presence of conc. H2SO4.
Answer
On warming, the mixture gives fruity smell. Acetic acid on heating with an alcohol and a dehydrating agent [conc. H2SO4] forms an ester-ethyl acetate.
Question 8(2017)
Draw the structural formula for –
(i) 2, 3 – dimethyl butane
(ii) Diethyl ether
(iii) Propanoic acid
Answer
(i) 2, 3 – dimethyl butane :

(ii) Diethyl ether :

(iii) Propanoic acid :

Question 1(2018)
Choose the correct answer from the options given:
(i) The organic compound which undergoes substitution reaction is:
A: C2H2
B: C2H4
C: C10H18
D: C2H6
(ii) The IUPAC name of dimethyl ether is:
A: Ethoxy methane
B: Methoxy methane
C: Methoxy ethane
D: Ethoxy ethane
Answer
(i) C2H6
(ii) Methoxy methane
Question 2(2018)
Give one word or a phrase for: The tendency of an element to form chains of identical atoms.
Answer
Catenation
Question 3(2018)
Give the IUPAC name for:
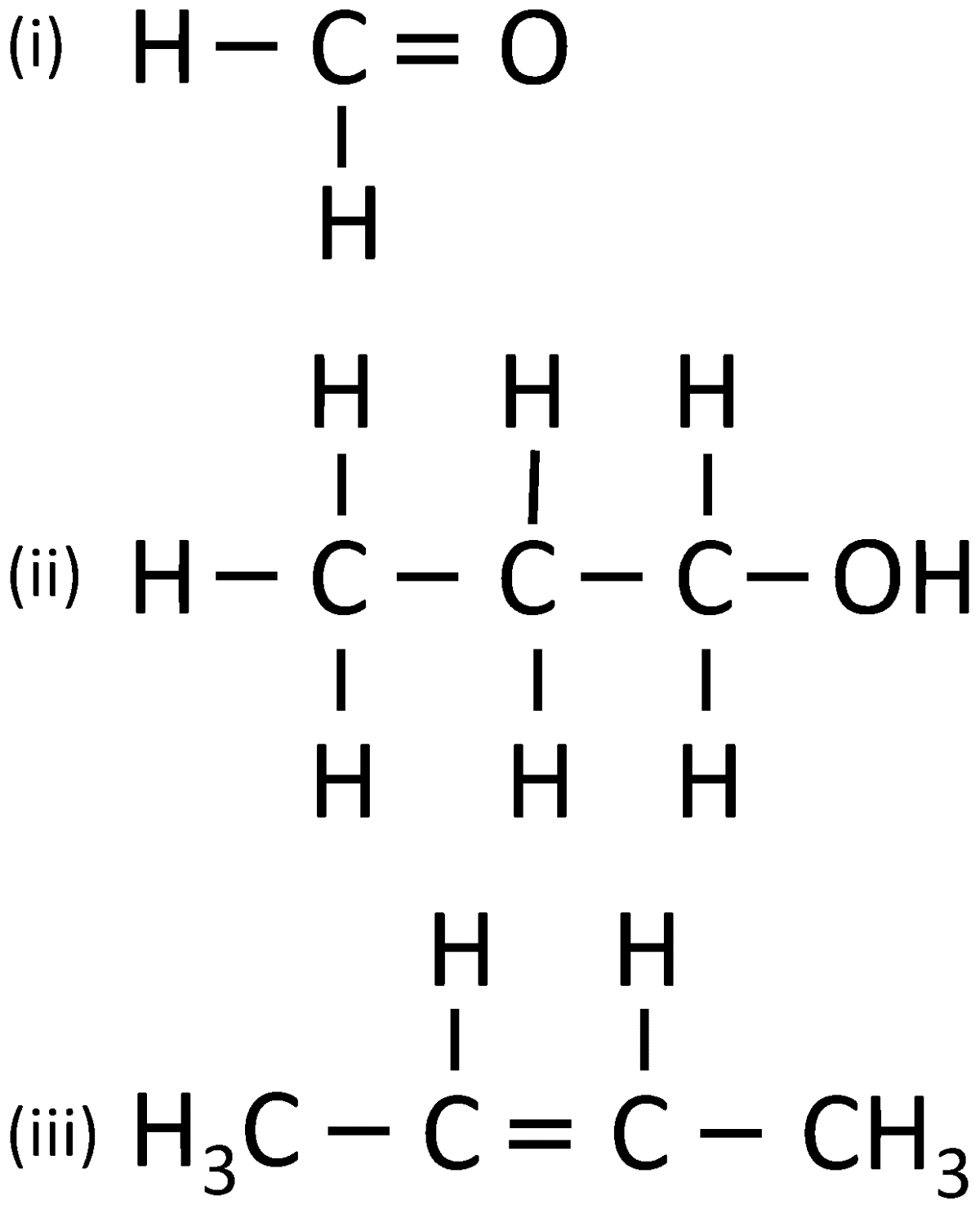
Answer
(i) Methanal
(ii) 1-Propanol
(iii) 2-Butene
Question 4 (2018)
Write the structural formula of the two isomers of butane.
Answer
Structural formula of the two isomers of butane are given below:
- Butane [n-butane]

- 2-Methyl propane [iso-butane]

Question 5(2018)
Name the gas that is produced during reaction of ethanol and sodium.
Answer
Hydrogen gas is produced during reaction of ethanol and sodium as shown below:
Question 6(2018)
Give the :
(i) IUPAC name of the homologous series.
(ii) Characteristic bond type.
(iii) IUPAC name of the first member of the series - in each case i.e., (a) and (b) relating to the homologous series of hydrocarbons - whose general formula is (a) CnH2n-2 (b) CnH2n+2
Answer
(a) Hydrocarbons with general formula CnH2n-2
- IUPAC name of the homologous series — Alkynes
- Characteristic bond type — Triple covalent bond
- IUPAC name of the first member of the series — Ethyne
(b) Hydrocarbons with general formula CnH2n+2
- IUPAC name of the homologous series — Alkanes
- Characteristic bond type — Single covalent bond
- IUPAC name of the first member of the series — Methane
Question 7(2018)
A compound X [having vinegar like smell] when treated with ethanol in the presence of the acid Z, gives a compound Y which has fruity smell. The reaction is
(i) Identify Y and Z
(ii) Write the structural formula of X.
(iii) Name the above reaction
Answer
(i) Y is ethyl acetate and Z is conc. sulphuric acid
(ii) Structural formula of X (ethanoic acid) is shown below:

(iii) Esterification reaction
Question 1(2019)
A hydrocarbon which is a greenhouse gas is:
A: Acetylene
B: Ethylene
C: Ethane
D: Methane
Answer
Methane
Question 2(2019)
Fill in the blanks with the choices given in the brackets:
(i) Conversion of ethanol to ethene by the action of conc. sulphuric acid is an example of ............... [dehydration/dehydrogenation/dehydrohalogenation]
(ii) Substitution reactions are characteristic reactions of ...............[alkynes/alkenes/alkanes].
Answer
(i) Conversion of ethanol to ethene by the action of conc. sulphuric acid is an example of dehydration
(ii) Substitution reactions are characteristic reactions of alkanes
Question 3(2019)
Write a balanced chemical equation for the following reactions: Chlorine gas is reacted with ethene.
Answer
The balanced chemical equation for the reaction of chlorine gas with ethene is given below:

Question 4(2019)
(i) Give the IUPAC name of the following organic compounds:
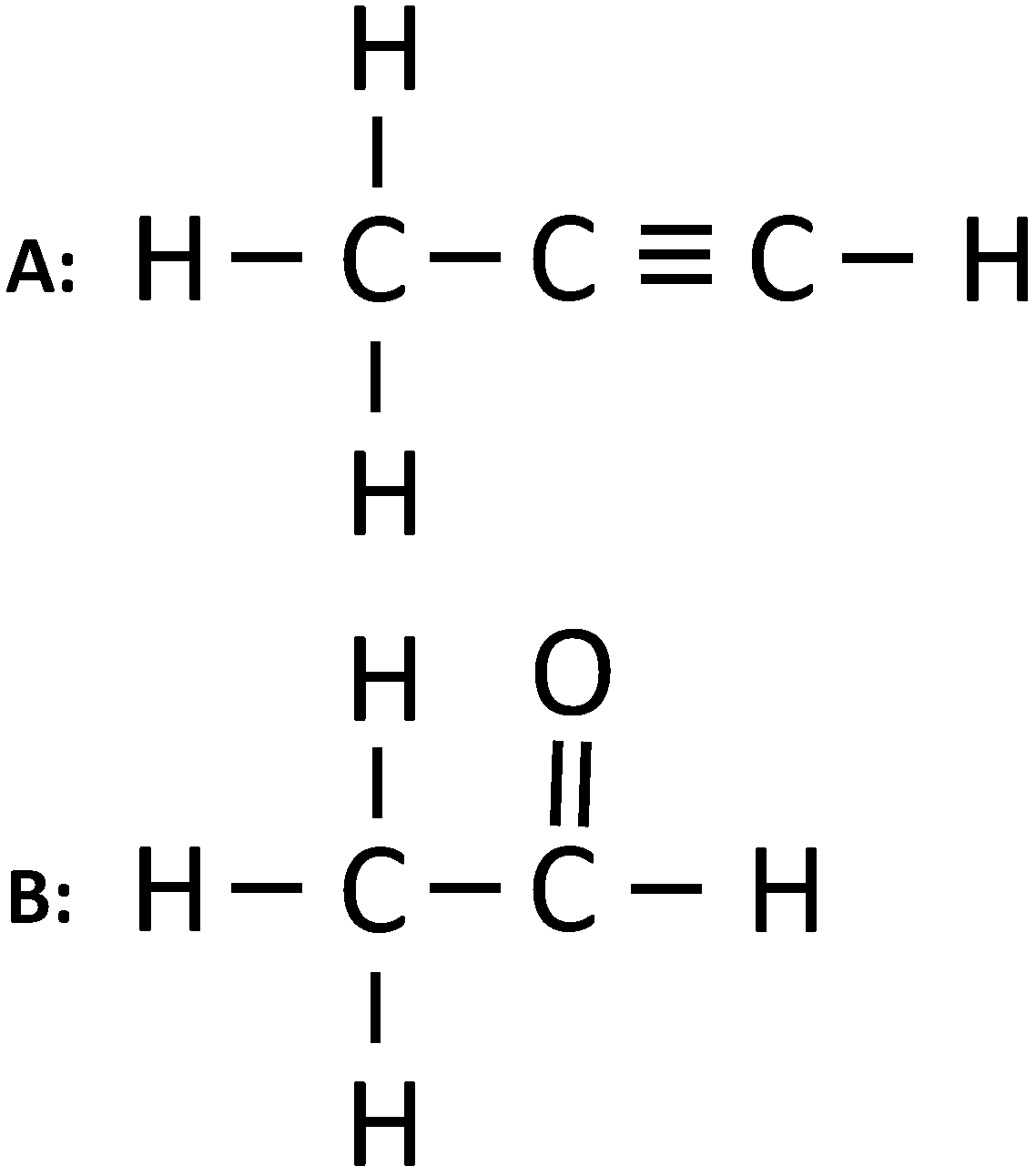
(ii) What is the special feature of the structure of ethyne
(iii) Name the saturated hydrocarbon containing two carbon atoms.
(iv) Give the structural formula of acetic acid.
Answer
(i) The IUPAC names are:
A: Propyne
B: Ethanal
(ii) The special feature of the structure of ethyne is that the two carbon atoms are linked by triple covalent bond formed by sharing three pairs of electrons between the two carbon atoms. The availability of electrons on the triple bond makes ethyne more reactive and hence it undergoes characteristics addition reactions only.
(iii) Ethane [C2H6]
(iv) Structural formula of acetic acid is shown below:

Question 5(2019)
Arrange - Ethane, methane, ethene, ethyne - in increasing order of molecular weight [H = 1, C = 12]
Answer
Ethane [C2H6] : M.W. = 2[12] + 6[1] = 30
Methane [CH4] : M.W. = 12 + 4[1] = 16
Ethene [C2H4] : M.W. = 2[12] + 4[1] = 28
Ethyne [C2H2] : M.W. = 2[12] + 2[1] = 26
Hence, increasing order of molecular weight :
Methane < Ethyne < Ethene < Ethane
Question 6(2019)
Give balanced chemical equations for the preparation of :
(i) Ethene from bromoethane
(ii) Ethyne using calcium carbide
(iii) Methane from sodium acetate.
Answer
(i) Ethene from bromoethane
(ii) Ethyne from calcium carbide
(iii)
Question 7(2019)
Name the following organic compounds:
(i) The compound with three carbon atoms whose functional group is a carboxyl.
(ii) The first homologue whose general formula is CnH2n
(iii) The compound that reacts with acetic acid to form ethyl ethanoate
(iv) The compound formed by complete chlorination of ethyne.
Answer
(i) Propanoic acid [C3H7CHO]
(ii) Ethene [C2H4]
(iii) Ethyl alcohol
(iv) 1,1,2,2 tetrachloroethane

Question 8(2019)
Identify the substances italicised : The organic compound which when solidified, forms an ice like mass.
Answer
Glacial acetic acid
Question 9(2019)
Name the gas evolved: Ethene undergoes hydrogenation reaction.
Answer
Ethane gas
Question 1(2020)
Choose the correct answer from the options: The organic compound having a triple carbon-carbon covalent bond, is
A: C3H4
B: C3H6
C: C3H8
D: C4H10
Answer
C3H4
Reason — C3H4 is an Alkyne as it follows the general formula CnH2n-2 and Alkynes contain a triple carbon-carbon covalent bond.
Question 2(2020)
State one relevant observation for the following reaction: A piece of sodium metal is put into ethanol at room temperature.
Answer
Effervesence of hydrogen gas is seen when a piece of sodium metal is put into ethanol at room temperature.
Question 3(2020)
Write a balanced chemical equation for each of the following:
(i) Producing ethane from bromoethane using Zn/Cu couple in alcohol.
(ii) Complete combustion of ethane
Answer
(i) Ethane from bromoethane using Zn/Cu couple in alcohol
(ii) Complete combustion of ethane
Question 4i(2020)
Draw the structural formula for each of the following:
A: 2,2 dimethyl pentane
B: methanol
Answer
A: 2,2 dimethyl pentane

B: methanol

Question 4ii(2020)
Write the IUPAC name for the following compounds:
A: acetaldehyde
B: acetylene
Answer
A: acetaldehyde — Ethanal
B: acetylene — Ethyne
Question 5(2020)
State one relevant reason for the following: Sodalime is preferred to sodium hydroxide in the laboratory preparation of methane.
Answer
Sodalime is preferred to sodium hydroxide in the laboratory preparation of methane since it is not deliquescent and does not attack glass.
Question 6(2020)
Give one word or a phrase for the following statement:
The reaction in which carboxylic acid reacts with alcohol in the presence of conc. H2SO4, to form a substance having a fruity smell.
Answer
Esterification
Question 7(2020)
Draw the structures of isomers of pentane.
Answer
The structures of isomers of pentane are shown below:
Pentane [n-pentane]

2-Methyl butane [iso-pentane]

2,2 Dimethyl Propane [neo-pentane]

Question 8(2020)
Copy and complete the following paragraph using the options given in brackets:
Alkenes are a homologous series of (i) ............... [saturated/unsaturated] hydrocarbons characterized by the general formula (ii) ............... [CnH2n+2/CnH2n]. Alkenes undergo (iii) ............... [addition/substitution] reactions and also undergo (iv) ............... [hydrogentaion/dehydrogenation] to form alkanes.
Answer
Alkenes are a homologous series of unsaturated hydrocarbons characterized by the general formula CnH2n. Alkenes undergo addition reactions and also undergo hydrogentaion to form alkanes.
Additional Questions
Question 1
Explain the term 'Organic Chemistry'. State the 'Natural sources' and 'Importance' of organic compounds.
Answer
Organic Chemistry is the study of carbon compounds excluding oxides of carbon, metallic carbonates and related compounds like metal cyanides, metal carbides.
Natural sources of organic compounds are:
- Plants
- Animals
- Coal
- Petroleum
- Fermentation
- Wood
Importance of organic compounds — Organic compounds are extremely useful to us in our daily life. The soaps and shampoos we use while taking bath, the powders, perfumes, we apply on the body, the clothes we wear, food we eat i.e., carbohydrates, proteins, fats, vitamins, etc., fuels we use, natural gas, petroleum products, medicines, explosives, dyes, insecticides, are all organic compounds.
There is hardly any walk of life where we do not need organic compounds.
Question 2
Explain the 'unique nature of carbon atom' with reference to —
(a) 'Tetravalency' — of carbon
(b) 'Catenation' — leading to formation of single, double and triple bonds and straight chain, branched chain and cyclic compounds.
Answer
(a) Tetravalency of carbon atoms — Carbon has four valence electrons. It forms four covalent bonds by mutually sharing it's four electrons with other atoms. Carbon is hence tetravalent or exhibits tetravalency. [At. no. of C = 6; Elec. Config. = 2,4]
(b) Catenation — Carbon atoms possess a unique property to link together (self linking) to form very long chains. This property is known as catenation. Catenation is shown by other elements also but Carbon exhibits this property of the maximum extent. This is due to the greater strength of carbon-carbon bond and due to tetra-covalency of carbon.
- Formation of single, double and triple covalent bonds — Carbon is tetravalent having a valency of four. In order to satisfy its valency, it forms single, double and triple covalent bonds by sharing one, two or three pairs of electrons respectively between two carbon atoms as well as with other atoms like oxygen, nitrogen and so on.

- Formation of straight chain, branched chain and cyclic compounds — The combination of carbon atoms with each other gives rise to straight or branched or cyclic and closed chains.

Question 3
State reasons for 'Justification of a separate branch' for 'Organic Chemistry'.
Answer
Due to unique nature of carbon atoms, which includes tetravalency, catenation and leading to formation of isomers, millions of organic compounds are known or synthesized [almost 90% of all known compounds are organic in nature]. Hence, a separate branch of chemistry called Organic Chemistry is required for the study of carbon and its compounds.
Question 4
State five differences between the characteristics of organic and inorganic compounds. State how organic compounds are classified.
Answer
Five differences between the characteristics of organic and inorganic compounds:
| Characteristic | Organic Compound | Inorganic Compound |
|---|---|---|
| Presence of carbon | Carbon is a necessary element in every organic compound. | Carbon is not an essential element in inorganic compounds. |
| Solubility in water | They generally do not dissolve in water. | They generally dissolve in water. |
| Solubility in organic solvents | They dissolve in organic solvents like alcohol, benzene and chloroform. | All inorganic compounds do not dissolve in organic solvents. |
| Melting and boiling point | They have low m.p. and b.p. and easily decompose on heating. | They have high m.p. and b.p. and usually do not decompose on heating. |
| Bonding | They form covalent bonds. | Most of them form ionic bonds. |
Classification of organic compounds:
| Aliphatic : Open chain compounds | Cyclic : Closed chain compounds |
|---|---|
| Hydrocarbons [compounds containing C & H only] | Homocyclic - [only C atoms] e.g., Aromatic compounds: Benzene |
| (a) Saturated - Alkanes e.g., Ethane | Heterocyclic [C,O,N,S atoms] e.g., Aromatic compounds - Pyridine |
| (b) Unsaturated - Alkenes e.g., Ethene, Alkynes e.g., Ethyne |
Question 5
Explain the term 'Homologous series'. State the general characteristics of members of the series with special reference to molecular mass or molecular formula.
Answer
A Homologous series is a group of organic compounds having a similar structure and similar chemical properties in which the successive compounds differ by a CH2 group.
General Characteristics of members of homologous series are:
- General Formula — All members of a homologous series share the same general formula. (Same elements and same functional group). For example, the general formula for alkane is CnH2n+2, alkene is CnH2n, alkyne is CnH2n-2.
- Molecular Mass — Each member of the series differs from the preceding one by the addition of CH2 group and by molecular mass of 14 a.m.u [CH2 = 12 + 2 = 14].
- Physical Properties — The physical properties of the members change gradually showing gradation in properties as the number of carbon atoms per molecule increases, i.e. as molecular mass increases.
For example, melting point, boiling point and the density of the successive members of the homologous series increase with increase in molecular mass. - Chemical Properties — Members of a homologous series have similar chemical properties.
For example, Methane reacts with chlorine to form methyl chloride:
CH4 + Cl2 ⟶ CH3Cl + HCl
Similarly, ethane reacts to form ethyl chloride
C2H6 + Cl2 ⟶ C2H5Cl + HCl - Preparation — All the members of a homologous series can be prepared by using the same general method of preparation.
Question 6
Differentiate between — 'Molecular formula' and 'Structural formula' — of an organic compound. Write the 'condensed structural formula' and 'branched structural formula' of ethene.
Answer
| Molecular Formula | Structural Formula |
|---|---|
| Indicates actual number of each kind of atoms in a molecule of the organic compound. | Indicates arrangement of various atoms in a molecule of the organic compound. |
| Gives no information about the type and number of bonds. | Chemical bonds are given along with the type of bonds for covalent bonds. |
Condensed structural formula of ethene:
H2C=CH2
Branched structural formula of ethene:

Question 7
State what are 'Alkyl groups'. State the alkyl group of the parent alkane — methane and ethane.
Answer
All groups derived from parent alkane by loss of 'H' atom are called alkyl groups.
Alkyl group of Methane is Methyl (CH3)
Alkyl group of Ethane is Ethyl (C2H5)
Question 8
State what are 'Functional groups'. Name the following functional groups —
C=C; -C≡C-; -OH; -CHO; -COOH; X = -F, -Cl, -Br, -I ; -C=O; -C-O-C-
with one example each of an organic compound with the respective functional group.
Answer
An atom, radical or bond which defines the structure of an organic compound and confers characteristic properties to it is known as a functional group.
| Group | Name | Example |
|---|---|---|
| C=C | carbon-carbon double bond | Ethene (C2H4) |
| -C≡C- | carbon-carbon triple bond | Ethyne (C2H2) |
| -OH | Hydroxyl | Methanol (CH3OH) |
| -CHO | Aldehydic | Methanal (HCHO) |
| -COOH | Carboxylic | Methanoic acid (HCOOH) |
| X = -F, -Cl, -Br, -I | Halo | Chloro Methane (CH3Cl) |
| -C=O | Ketonic | Propanone (CH3-CO-CH3) |
| -C-O-C- | Alkoxy | Methoxy Methane (CH3-O-CH3) |
Question 9
Explain the terms — 'Isomers' and 'Isomerism'. State the 'Characteristics of isomers' with reference to —
- Properties of isomers
- Number of isomers with relation to carbon atoms in the isomer.
Differentiate between — 'Chain isomerism' and 'Position isomerism ' — with suitable examples.
Answer
Isomers & Isomerism — Organic compounds having the same molecular formula but differing in molecular arrangement or in structural formula are called Isomers and the phenomenon is called Isomerism.
Characteristics of Isomers:
- Properties of Isomers — Isomers of the same homologous series have similar chemical properties but differ in physical properties.
- Number of Isomers — Number of Isomers increases with increase in number of carbon atoms. [e.g., C4H10 has two isomers, C5H12 has three isomers]
Difference between Chain Isomerism and Position Isomerism:
Chain Isomerism occurs due to the difference in arrangemnet of Carbon atoms in the chain whereas Position Isomerism occurs due to the difference in position of functional group.
Isomers of Pentane exhibit chain isomerism:
n-pentane

iso-pentane

Isomers of Butyne exhibit position isomerism:
but-1-yne

but-2-yne

Question 10
Explain the term – 'Nomenclature'. State it's need with reference to organic compounds. State the basic rules of Nomenclature by the trivial system with suitable examples. Explain the longest chain rule and the smallest number for functional groups rule of Nomenclature by the IUPAC system with suitable examples.
Answer
Nomenclature is the system of assignment of names to organic compounds.
Need for Nomenclature — Large number of organic compounds due to:
- Varying molecular structures of organic compounds.
- Isomerism in organic compounds
increases need for correct, methodical and systematic naming of each compound.
Basic rules of Nomenclature by the trivial system:
The basis of naming organic compounds by the trivial system is its :
- Source
- Properties
- Latin or greek origin of compounds
For example,
Methane (CH4) was named marsh gas since it was obtained from marshy places.
The name Acetic acid is derived from it's source 'vinegar' [Latin : acetum]
Longest Chain Rule — The longest continuous chain of 'C' atoms, known as parent chain is selected. The longest chain need not be straight.
For example,
(i) The longest chain is of 5 carbon atoms, so root word is 'pent'

(ii) Longest chain is of 7 carbon atoms, so the root word is 'hept'. The reamining carbon atom (unnumbered) is considered a branch.

Smallest number for functional groups rule — In case, any functional group is also present in the chain, then the carbon atom are numbered in such a way that the functional group gets the smallest possible number.
For example,

Question 11
Explain the term – 'Hydrocarbons'. State the two main groups of hydrocarbons with examples. Draw a chart differentiating — 'Alkanes, Alkenes and Alkynes' — with respect to:
- General formula
- Characteristic bond
- IUPAC and the common name of the first three members and condensed/branched/electronic structural formula of each
- Availability of electrons
- Reactivity
- Characteristic reaction.
Answer
Hydrocarbons — Hydrocarbons are compounds that are made up only of carbon and hydrogen atoms.
Classification of Hydrocarbons — Hydrocarbons are sub-divided into two main groups, the aliphatic (open) and cyclic (closed) chain compounds. The open chain compounds or aliphatic hydrocarbons are further sub-divided into saturated hydrocarbons and unsaturated hydrocarbons. Example of saturated hydrocarbons is the homologous series of Alkanes. Examples of unsaturated hydrocarbons are the homologous series of Alkenes and Alkynes.
Question 12
Draw the structural formula of each of the following :
| Alkane | Alkene | Alkyne |
|---|---|---|
| (a) Methane | (a) No corresponding alkene | (a) No corresponding alkyne |
| (b) Ethane | (b) Ethene | (b) Ethyne |
| (c) Propane | (c) Propene | (c) Propyne |
| (d) Butane -chain isomers (i) 1-butane [n-butane] (ii) 2-methyl propane [iso-butane] | (d) Butene -Position isomers (i) 1-butene (ii) 2-butene - Chain isomer (i) 2-methyl prop-1-ene | (d) Butyne -Position isomers (i) 1-butyne (ii) 2-butyne |
| (e) Pentane - chain isomers (i) 1-pentane [n-pentane] (ii) 2-methyl butane [iso-pentane] (iii) 2-2-dimethylpropane [neo-pentane] | Pentene - Position isomers (i) 1-pentene (ii) 2-pentene -Chain isomer (i) 2-methyl but-1-ene (ii) 3-methyl but-1-ene | (e) Pentyne - Position isomers (i) 1-pentyne (ii) 2-pentyne -Chain isomer 3-methyl but-1-yne |
| Alcohols | Aldehydes | Carboxylic acids |
|---|---|---|
| (a) Methanol [methyl alcohol] | (a) Methanal [formaldehyde] | (a) Methanoic acid [formic acid] |
| (b) Ethanol [ethyl alcohol] | (b) Ethanal [acetaldehyde] | (b) Ethanoic acid [acetic acid] |
| (c) Propanol - Position isomers (i) 1-propanol [n-propylalcohol] (ii) 2-propanol [iso-propylalcohol] | (c) Propanal [propionaldehyde] | (c) Propanoic acid [propionic acid] |
| (d) Butanol - Position isomers (i) 1-butanol (ii) 2-butanol - Chain isomer (i) 2-methyl propan 1 -ol (ii) 2-methyl propan 2 -ol | (d) Butanal [butraldehyde] | (d) Butanoic acid [butyric acid] - Chain isomer (i) 2-methyl propanoic acid [iso-butyriac acid] |
| (e) Pentanol -Position isomers (i) 1-pentanol (ii) 2-pentanol (iii) 3-pentanol |
| Ketones | Alkyl Halides | Ether | Ester |
|---|---|---|---|
| (a) Propanone [acetone] | (a) Monochloro methane [methyl chloride] | (a) Methoxy methane [dimethyl ether] | (a) Methyl methanoate [methyl formate] |
| (b) 2-Butanone [ethyl methyl ketone] | (b) Bromoethane [ethyl bromide] | (b) Methoxy ethane [ethyl methyl ether] | (b) Methyl ethanoate [methyl acetate] |
| (c) 3-Pentanone [diethyl ketone] | (c) Iodomethane [methyl iodide] | (c) Ethoxy ethane [diethyl ether] | (c) Ethyl ethanoate [ethyl acetate] |
Answer
The structural formulae are shown below:
Alkane
(a) Methane

(b) Ethane

(c) Propane

(d) Butane - Chain isomers
(i) 1-butane (n-butane)

(ii) 2-methyl propane [iso-butane]

(e) Pentane - Chain isomers
(i) 1-pentane [n-pentane]

(ii) 2-methyl butane [iso-pentane]

(iii) 2-2-dimethylpropane [neo-pentane]

Alkene
(b) Ethene

(c) Propene

(d) Butene - Position isomers
(i) 1-butene

(ii) 2-butene

(d) Butene - Chain isomers
(i) 2-methyl prop-1-ene

(e) Pentene - Position isomers
(i) 1-pentene

(ii) 2-pentene

(e) Pentene - Chain isomers
(i) 2-methyl but-1-ene

(ii) 3-methyl but-1-ene

Alkyne
(b) Ethyne

(c) Propyne

(d) Butyne - Position isomers
(i) 1-butyne

ii) 2-butyne

(e) Pentyne — Position isomers
(i) 1-pentyne

(ii) 2-pentyne

(e) Pentyne — Chain isomer
(i) 3-methyl but-1-yne

Alcohol
(a) Methanol [methyl alcohol]

(b) Ethanol [ethyl alcohol]

(c) Propanol — Position isomers
(i) 1-propanol [n-propylalcohol]

(ii) 2-propanol [iso-propylalcohol]

(d) Butanol — Position isomers
(i) 1-butanol

(ii) 2-butanol

(d) Butanol — Chain isomers
(i) 2-methyl propan-1-ol

(ii) 2-methyl propan-2-ol

(e) Pentanol — Position isomers
(i) 1-pentanol

(ii) 2-pentanol

(iii) 3-pentanol

Aldehydes
(a) Methanal [formaldehyde]

(b) Ethanal [acetaldehyde]

(c) Propanal [propionaldehyde]

(d) Butanal [butraldehyde]

Carboxylic acids
(a) Methanoic acid [formic acid]

(b) Ethanoic acid [acetic acid]

(c) Propanoic acid [propionic acid]

(d) Butanoic acid [butyric acid] - Chain isomer
(i) 2-methyl propanoic acid [iso-butyriac acid]

Ketones
(a) Propanone [acetone]

(b) 2-Butanone [ethyl methyl ketone]

(c) 3-Pentanone [diethyl ketone]

Alkyl Halides
(a) Monochloro methane [methyl chloride]

(b) Bromoethane [ethyl bromide]

(c) Iodomethane [methyl iodide]

Ether
(a) Methoxy methane [dimethyl ether]

(b) Methoxy ethane [ethyl methyl ether]

(c) Ethoxy ethane [diethyl ether]

Ester
(a) Methyl methanoate [methyl formate]

(b) Methyl ethanoate [methyl acetate]

(c) Ethyl ethanoate [ethyl acetate]

Question 13
Give the IUPAC name of the compounds numbered (i) to (v).

Answer
(i) 3-Methyl butanol
(ii) 2, 2-dimethyl propanol
(iii) 3-Bromo 2-methyl butanoic acid
(iv) 3-Methyl butanal
(v) 3-Methyl butan 2-one
Question 14
Give balanced equations for the laboratory preparations of - Alkanes, Alkenes, Alkynes, Alcohols and Acids.
| Sl. No. | Compound | from: | by/from: |
|---|---|---|---|
| (a) | Methane [CH4] | Sodium ethanoate [sodium acetate] | by -decarboxylation |
| (b) | Methane [CH4] | Iodo methane [methyl iodide] | from -an alkylhalide |
| (c) | Ethane [C2H6] | Sodium propanoate [sodium propionate] | by -decarboxylation |
| (d) | Ethane [C2H6] | Bromo ethane [ethyl bromide] | from -alkylhalide |
| (e) | Ethene [C2H4] | from ethanol [ethyl alcohol] | by -dehydration |
| (f) | Ethene [C2H4] | Bromo ethane [ethyl bromide] | by - dehydrohalogenation |
| (g) | Ethyne [C2H2] | from calcium carbide | from -a calcium compound |
| (h) | Ethyne [C2H2] | 1,2, dibromoethane [ethylene dibromide] | by -dehydrohalogenation |
| (i) | Ethanol [C2H5OH] | Bromo ethane [ethyl bromide] | by - hydrolysis of alkylhalide |
| (j) | Ethanol [C2H5OH] | Ethene [ethylene] | by - hydration of ethene |
| (k) | Ethanoic acid [CH3COOH] | Ethanol [ethyl alcohol] | by - oxidation of alcohol |
Answer
(a) Methane [CH4] by decarboxylation :
(b) Methane [CH4] from alkyl halide :
(c) Ethane [C2H6] by decarboxylation :
(d) Ethane [C2H6] from alkylhalide :
(e) Ethene [C2H4] by dehydration :
(f) Ethene [C2H4] by dehydrohalogenation :
(g) Ethyne [C2H2] from Calcium Carbide :
(h) Ethyne [C2H2] from 1,2, dibromoethane [ethylene dibromide] by -dehydrohalogenation :

(i) Ethanol [C2H5OH] by hydrolysis of alkylhalide:
(j) Ethanol [C2H5OH] by hydration of ethene:
(k) Ethanoic acid [CH3COOH] by oxidation of alcohol :
Question 15.1
Give equations for the conversions of – Methane, Ethane:
| Methane to | Ethane to | |
|---|---|---|
| (a) Carbon tetrachloride | (a) Hexachloro ethane | by - Substitution [diffused sunlight] |
| (b) Carbon dioxide | (b) Carbon dioxide | by - Oxidation - Complete |
| (c) Methanol to Methanal to Methanoic acid | Ethanol to Ethanal to Ethanoic acid | by - Oxidation (i) catalytic using - catalyst copper [Cu] (ii) Controlled using acidified K2Cr2O7 |
| (d) Methnal | Ethanal | by- Oxidation - Catalytic - using catalyst MoO |
| (e) Ethyne | Ethene | by - Pyrolysis [dehydrogenation] |
Answer
(a) Conversion of Methane to Carbon tetrachloride by substitution [diffused sunlight]:
(b) Conversion of Methane to Carbon dioxide by complete oxidation:
(c)(i) Conversion of Methane to Methanol to Methanal to Methanoic acid by catalytic oxidation using catalyst copper [Cu]:
(c)(ii) Conversion of Methane to Methanol to Methanal to Methanoic acid by controlled slow oxidation using acidified K2Cr2O7:
(d) Conversion of Methane to Methanal by catalytic oxidation using catalyst MoO:
(e) Conversion of Methane to Ethyne by Pyrolysis [dehydrogenation]:
(a) Conversion of Ethane to Hexachloro ethane by substitution [diffused sunlight]:
(b) Conversion of Ethane to Carbon dioxide by complete oxidation:
(c)(i) Conversion of Ethane to Ethanol to Ethanal to Ethanoic acid by catalytic oxidation using catalyst copper [Cu]:
(c)(ii) Conversion of Ethane to Ethanol to Ethanal to Ethanoic acid by controlled slow oxidation using acidified K2Cr2O7:
(d) Conversion of Ethane to Ethanal by catalytic oxidation using catalyst MoO:
(e) Conversion of Ethane to Ethene by Pyrolysis [dehydrogenation]:
Question 15.2
Give equations for the conversions of – Ethene, Ethyne to:
| Ethene | Ethyne | |
|---|---|---|
| (a) Ethane | (a) Ethane [2 steps ] | by - Catalytic hydrogenation - H2 |
| (b) 1,2 dichloroethane | (b) 1,1,2,2 tetrachloroethane [2 steps] | by- Halogenation - Cl2 |
| (c) 1,2 dibromoethane | (c) 1,1,2,2 tetrabromoethane [2 steps] | - Br2 |
| (d) 1,2 diiodoethane | (d) 1,2 diiodoethene | - I2 |
| (e) Bromoethane/Chloroethane | (e) 1,1 -dibromo & 1,1-dichloroethane | by- Halogen acids - HBr/HCl by - Polymerization |
| (f) Polyethylene | (f) Copper or silver Acetylide | by Ammoniacal - CuCl/AgNO3 |
Answer
(a) Conversion of Ethene to Ethane by Catalytic hydrogenation (H2):
(b) Conversion of Ethene to 1,2 dichloroethane by Halogenation (Cl2):

(c) Conversion of Ethene to 1,2 dibromoethane by Halogenation (Br2):

(d) Conversion of Ethene to 1,2 diiodoethane by Halogenation (I2):
(e)(i) Conversion of Ethene to Bromoethane by Halogen acids (HBr):
(e)(ii) Conversion of Ethene to Chloroethane by Halogen acids (HCl):
(f) Conversion of Ethene to Polyethylene by Polymerization:
Ethene polymerises to produce polythene
(a) Conversion of Ethyne to Ethane [2 steps] by Catalytic hydrogenation (H2):
![Give equation for the conversion of Ethyne to Ethane [2 steps] by Catalytic hydrogenation. Organic Chemistry, Simplified Chemistry Dalal Solutions ICSE Class 10.](https://cdn1.knowledgeboat.com/img/scd10/ethyne-hydrogenation-organic-chemistry-icse-class-10-1200x298.png)
(b) Conversion of Ethyne to 1,1,2,2 tetrachloroethane [2 steps] by Halogenation (Cl2):
![Give equation for the conversion of Ethyne to 1,1,2,2 tetrachloroethane [2 steps] by Halogenation. Organic Chemistry, Simplified Chemistry Dalal Solutions ICSE Class 10](https://cdn1.knowledgeboat.com/img/scd10/ethyne-chlorine-reaction-organic-chemistry-icse-class-10-1200x371.png)
(c) Conversion of Ethyne to 1,1,2,2 tetrabromoethane [2 steps] by Halogenation (Br2):
![Give equation for the conversion of Ethyne to 1,1,2,2 tetrabromoethane [2 steps] by Halogenation. Organic Chemistry, Simplified Chemistry Dalal Solutions ICSE Class 10](https://cdn1.knowledgeboat.com/img/scd10/ethyne-bromine-reaction-organic-chemistry-icse-class-10-1200x368.png)
(d) Conversion of Ethyne to 1,2 diiodoethene by Halogenation (I2):
(e)(i) Conversion of Ethyne to 1,1 dibromo ethane by Halogen acids (HBr):

(e)(ii) Conversion of Ethyne to 1,1 dichloro ethane by Halogen acids (HCl):

(f)(i) Conversion of Ethyne to Copper Acetylide by Ammoniacal Cuprous Chloride:
(f)(ii) Conversion of Ethyne to Silver Acetylide by Ammoniacal Silver Nitrate:
Question 15.3
Give equations for the conversions of – Ethanol and Ethanoic Acid to:
| Ethanol [ethyl alcohol] | Ethanoic Acid [acetic acid] |
|---|---|
| (a) Carbon dioxide - by burning | (a) Sodium acetate - using alkali - NaOH |
| (b) Ethanal to Ethanoic acid - by oxidation using acidified K2Cr2O7 | (b) Calcium acetate - using alkali - Ca(OH)2 |
| (c) Sodium ethaoxide - using - sodium | (c) Ammonium acetate - using alkali - NH4OH |
| (d) Ethyl ethanoate - using - ethanoic acid and conc. H2SO4 | (d) Ethyl ethanoate -using - ethanol and conc. H2SO4 [ethyl acetate] |
| (e) Ethene - using conc. H2SO4 at 170 °C |
Answer
(a) Conversion of Ethanol to Carbon dioxide by burning:
(b) Conversion of Ethanol to Ethanal to Ethanoic acid by oxidation using acidified K2Cr2O7:
(c) Conversion of Ethanol to Sodium ethoxide using sodium:
(d) Conversion of Ethanol to Ethyl ethanoate using ethanoic acid and conc. H2SO4:
(e) Conversion of Ethanol to Ethene using conc. H2SO4 at 170°C:
(a) Conversion of Ethanoic acid [acetic acid] to Sodium acetate using alkali - NaOH:
(b) Conversion of Ethanoic acid [acetic acid] to Calcium acetate using alkali - Ca(OH)2:
(c) Conversion of Ethanoic acid [acetic acid] to Ammonium acetate using alkali - NH4OH:
(d) Conversion of Ethanoic acid [acetic acid] to Ethyl ethanoate [ethyl acetate] using ethanol and conc. H2SO4:
Question 16
Give reasons for:
(i) alkanes are said to be saturated organic compounds
(ii) alkenes are known as olefins
(iii) alkenes are more reactive than alkanes
(iv) ethanoic acid is known as an aliphatic monocarboxylic acid.
Answer
(i) In alkanes, all the four valencies of each carbon atom are satisfied by the hydrogen atoms, forming single covalent bond. The non-availability of electrons in the single covalent bond makes them less reactive and therefore they undergo substitution reaction only. Hence, they are called saturated hydrocarbon.
(ii) Alkenes are known as olefins [Oleum = oil; ficare = to make] because they form oily products on treatment with halogens (like chlorine or bromine).
(iii) Alkenes are unsaturated hydrocarbons having carbon atoms forming a double covalent bond as their valencies are not fully satisfied by hydrogen atoms whereas alkanes are saturated hydrocarbons as all the four valencies of its carbon atoms are satisfied by the hydrogen atoms. The availability of electrons in the double bond in case of alkenes makes them more reactive than alkanes which has do not have electrons available in the single covalent bond.
(iv) Ethanoic acid (CH3 – COOH) contains only one – COOH group (carboxylic acid group) that is why it is called a monocarboxylic acid. As ethanoic acid does not contain a benzene ring, so it is an aliphatic monocarboxylic acid.
Question 17
Explain the terms –
(i) Denaturated alcohol
(i) Glacial acetic acid
(iii) Esterification
Answer
(i) Ethyl alcohol containing pyridine or copper sulphate is termed denaturated alcohol. It is used for industrial applications only and hence made undrinkable.
(ii) Anhydrous acetic acid on cooling below 16.5°C, crystallizes out in the pure form, forming a crystalline mass resembling ice [m.p. around 17°C] . Hence, pure acetic acid is called glacial acetic acid.
(iii) Esterification is the chemical process of heating carboxylic acids with alcohol in the presence of an acid catalyst to produce esters.
Question 18(i)
Give a chemical test to distinguish between Ethane, ethene and ethyne.
Answer
Chemical tests to distinguish between Ethane, Ethene and Ethyne:
| Sl. No. | Test | Ethane | Ethene | Ethyne |
|---|---|---|---|---|
| 1. | On adding a few drops of bromine solution in carbon tetrachloride (CCl4) to the hydrocarbon | No change is observed. | The reddish brown colour of bromine solution get decolourised. | The reddish brown colour of bromine solution get decolourised. |
| 2. | On adding a few drops of ammoniacal cuprous chloride (CuCl2) to the hydrocarbon | No change is observed. | No change is observed. | Red precipitate of copper acetylide is formed. |
Question 18(ii)
Give a chemical test to distinguish between Ethanol and ethanoic acid.
Answer
Add Sodium Carbonate (Na2CO3) / Sodium Hydrogen Carbonate (NaHCO3) solution to Ethanol and ethanoic acid. With Ethanol it will produce brisk effervescence of carbon dioxide (CO2) gas whereas no effervescence is seen with Ethanol.
The chemical equation for reaction between Sodium Carbonate (Na2CO3) / Sodium Hydrogen Carbonate (NaHCO3) and Ethanoic Acid is given below:
2CH3COOH + Na2CO3 ⟶ 2CH3COONa + H2O + CO2↑
CH3COOH + NaHCO3 ⟶ CH3COONa + H2O + CO2↑
Question 19
Give the main uses of –
(i) Methane
(ii) Ethane
(iii) Ethene
(iv) Ethyne
(v) Ethanol
(vi) Ethanoic acid.
Answer
(i) Uses of Methane:
- Methane is used as a domestic fuel.
- Methane is used in the preparation of useful compounds like ethyne (acetylene), methanal (formal-dehyde), methanol, chloro-methane and tetrachloro-methane (carbon tetrachloride).
- Methane is a source of carbon monoxide and hydrogen.
(ii) Uses of Ethane:
- Ethane is used as a gaseous and automobile fuel.
- Ethane is used in the preparation of ethene, ethanol, ethanal (acetaldehyde) and ethanoic acid (acetic acid).
- It forms ethyl chloride which is used to make tetraethyllead. 1, 1, 1-trichloroethane is one solvent that is used a lot in dry cleaning.
(iii) Uses of Ethene:
- Production of oxy-ethylene torch — For welding purposes and cutting metals.
- Ripening of green fruits — Artificial ripening and preservation of fruits.
- Catalytic hydrogenation — Used in hardening of oils.
- Manufacture of —
- Synthetic chemicals — Ethylene glycol [anti-freeze], di-ethyl ether [solvent], ethylene oxide [fumigant], mustard gas [chemical warfare],
- Polymers — Polyetheylene, polyvinyl chloride [P.V.C.]- used in packaging, insulators, containers, rain coats etc.
(iv) Uses of Ethyne:
- Production of oxy-acetylene torch — for welding and cutting metals [flame temp. 3500°C] .
- Ripening of green fruits — Artificial ripening and preservation of fruits.
- Manufacture of organic compounds — Acetic acid, Acetaldehyde, Acetylene dichloride, Ethyl alcohol, Oxalic acid.
- Manufacture of synthetic products — Polymers, synthetic rubbers and fibres.
(v) Uses of Ethanol:
- It is a good solvent for gums and resins.
- It is used in thermometers and as a preservative for biological specimens due to its low freezing point.
- It is used in the manufacture of chemicals such as chloroform, iodoform, ether, acetic acid and synthetic products like dyes, esters (perfumes), antiseptics, preservatives.
- Ethyl alcohol is used in alcoholic drinks like whisky, wine and beer.
- As a fuel in powdered form and as an antifreeze for automobile radiators.
(vi) Uses of Ethanoic acid:
- In manufacture of important organic compounds — Vinyl acetate [used in resins], acetic anhydride [used in aspirin], cellulose acetate [used in synthetic fibres], various dyes, perfumes and medicines.
- In the food industry — Vinegar for preserving and flavouring food.
- As a solvent — It dissolves phosphorous, sulphur, iodine, resins, cellulose, etc.
- As a laboratory reagent — It is used for preparing acetone, esters, etc.
- As a coagulant — For coagulating rubber from latex.





